Kidney involvement is one of the most severe forms of lupus and is often life-threatening. In order to detect lupus nephritis (LN) at an early stage, a kidney biopsy is recommended for SLE patients with abnormalities in the urine. Treatment options have improved thanks to the availability of modern add-on substances. In the KDIGO guideline published at the beginning of 2024, combination therapy with the addition of belimumab or voclosporin is recommended for class 3 or 4 LN. It is important to use an evidence-based treatment regimen.
Lupus nephritis (LN) occurs in up to half of all patients with systemic lupus erythematosus (SLE) [1]. “Patients with lupus nephritis have a significantly worse outcome,” reported Prof. Dr. med. Julia Weinmann-Menke, University Medical Center of Johannes Gutenberg University Mainz [2]. In a population-based study published in 2019, all patients diagnosed with SLE in Oslo in the period 1999-2008 (n=325) were followed up over a median follow-up period of 14 years. The mortality rate for LN patients was 3.8 compared to 1.7 for SLE patients without LN (95% CI: 2.1-6.2 and 0.9-2.7, respectively) [3]. However, treatment options for LN have improved in recent years and it is hoped that this will also be reflected in treatment outcomes. In addition to improving life expectancy, the most important treatment goals are the best possible organ protection and alleviation of individually varying symptoms. Early diagnosis of LN is a prerequisite for providing patients with adequate treatment. Accordingly, patients with known or suspected SLE should be routinely screened by urine sediment analysis as well as UPCR and UACR. If the presence of LN is confirmed in a subsequent kidney biopsy, the aim is to achieve and maintain remission as quickly as possible [2]. The KDIGO guideline published this year incorporates the latest evidence-based findings on the treatment of LN [4]. The approval of belimumab and voclosporin by the FDA, EMA and Swissmedic as an add-on to the standard treatment of LN represents a significant expansion of the therapeutic armamentarium and entails an adjustment of the existing treatment regimens [5–8]. Adequate basic therapy and, if necessary, immunosuppressive treatment are still important, emphasized Prof. Weinmann-Menke [2].
| Abbreviations |
| CKD = Chronic Kidney Disease |
| EMA = European Medicines Agency |
| FDA = US Food and Drug Administration |
| KDIGO = Kidney Disease: Improving Global Outcomes |
| UACR=Albumin/Creatinine Ratio |
| UPCR = Urine protein/creatinine ratio |
| SGLT-2 = Sodium-Glucose-Co-Transporter-2 |
Biopsy early and consider modern add-on substances if necessary
“We biopsy when we see abnormalities in the urine,” says the speaker [2]. This procedure corresponds to international guideline recommendations [1,4]. If LN is suspected on the basis of UPCR and UACR, a kidney biopsy with assessment of the histopathological class should be performed [1,4]. If class 3 or 4 LN is diagnosed, the KDIGO guideline recommends adding belimumab or voclosporin “on top” of the standard treatment right from the start [4]. “Every relapse of lupus nephritis leads to the loss of kidney tissue and thus worsens the overall survival of the patient,” explained Prof. Weinmann-Menke [2]. Belimumab (Benlysta®) has been approved for systemic lupus without LN for many years, so that the long-term effects and side effect profile are known [8]. In the BLISS-LN study, which is relevant for the approval of belimumab in LN, belimumab not only increased the response to therapy, but also reduced the loss of eGFR and the relapse rate [6]. And in the AURORA 1 study, more patients achieved a complete response in the treatment arm with voclosporin than without this additional therapy [9]. Voclosporin (Lupkynis®) received Swissmedic approval for LN in 2023 [8].
Do not neglect the “standard of care”
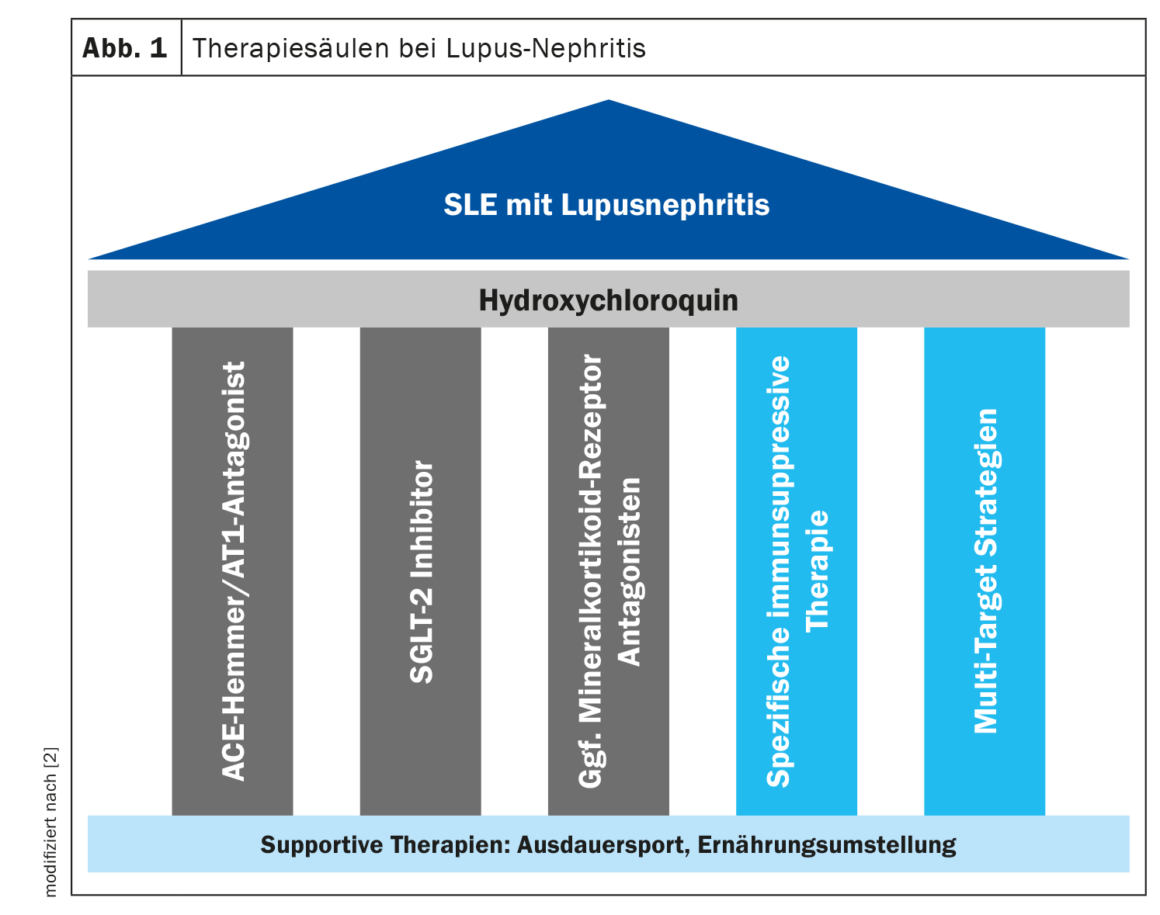
The treatment of LN consists of several therapeutic pillars (Fig. 1). “Hydroxychloroquine should be given to every patient with lupus nephritis,” said the speaker [2]. It has been shown that LN patients treated with hydroxychloroquine (HCQ) achieve significantly better outcomes; for example, observational studies have demonstrated an improvement in renal response rates, a reduction in the risk of relapse and the prevention of progression of CKD [10,11]. Retinal toxicity is a known side effect of long-term use of HCQ, which is why an ophthalmologic examination is recommended 5 years after the start of therapy and every year thereafter [1].
Proven pillars of basic therapy are the blockade of the renin-angiotensin-aldosterone system (RAAS) and the use of an SGLT-2 inhibitor [2]. RAAS blockade is indicated due to its antiproteinuric and antihypertensive effects, and SGLT-2-i has demonstrated nephroprotective, prognosis-improving effects in the large DAPA-CKD and EMPA-Kidney studies in CKD. “The lupus nephritis patient benefits if he receives the SGLT-2-i as an add-on,” said the speaker [2]. While LN classes I and II usually only require conservative therapy, immunosuppressive treatment is required for classes III and IV, and in some cases for class V [1]. Immunosuppressive induction therapy includes mycophenolate mofetil (MMF) or cycylophosphamide and steroid treatment at the lowest possible dose. Prof. Weinmann-Menke reported that KDIGO has a new scheme for reducing steroids [2,4]. The aim is to use the lowest possible steroid dose in order to reduce the side effects of long-term use, but at the same time not to jeopardize the maintenance of remission. A study published in 2023 showed that LN patients who suffered one or more flares had a more rapid decline in kidney function and a higher mortality rate [10]. The speaker therefore advises that steroids should only be discontinued in very stable patients who show a good response to therapy.
If remitted LN patients no longer show any abnormalities in their urine, it still makes sense to subject them to a further kidney biopsy after one year to see how their kidney activity is progressing. The further treatment procedure can then be determined on this basis.
Congress: DGIM Annual Conference
Literature:
- Odler B, et al.: Diagnostik und Therapie der Lupusnephritis – 2023 [Diagnostic and therapy of lupus nephritis – 2023]. Wien Klin Wochenschr 2023; 135(Suppl 5): 675–687.
- «Lupusnephritis: Highlights – Was ist neu für die Praxis», Univ.-Prof. Dr. med. Julia Weinmann-Menke, 130. Kongress der Deutschen Gesellschaft für Innere Medizin (DGIM), 13.04.2024.
- Reppe Moe SE, et al.: Assessing the relative impact of lupus nephritis on mortality in a population-based systemic lupus erythematosus cohort. Lupus 2019; 28(7): 818–825.
- Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int 2024; 105(1S): S1–S69.
- Rovin BH, et al.: Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Management of Lupus Nephritis. Kidney Int 2024; 105(1): 31–34.
- Furie R, et al.: Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020; 383: 1117–1128.
- Rovin BH, et al.: Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021; 397: 2070–2080.
- Swissmedic : Arzneimittelinformation,
www.swissmedicinfo.ch,(last retrieval 31.05.2024) - «Behandlung von PatientInnen mit aktiver Lupusnephritis nach Marktrücknahme von Voclosporin», Deutsche Gesellschaft für Rheumatologie (DGRh), https://dgrh.de, (letzter Abruf 31.05.2024)
- Peña-Vizcarra ÓR, et al.: Effect of antimalarials on clinical outcomes in lupus nephritis. Rheumatology (Oxford). 2023 Nov 1:kead576.
doi: 10.1093/rheumatology/kead576. - Kostopoulou M, et al.: Management of lupus nephritis: a systematic literature review informing the 2019 update of the joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations. RMD Open. 2020;6: 2. doi: 10.1136/rmdopen-2020-001263.
HAUSARZT PRAXIS 2024; 19(6): 24–25 (published on 26.6.24, ahead of print)
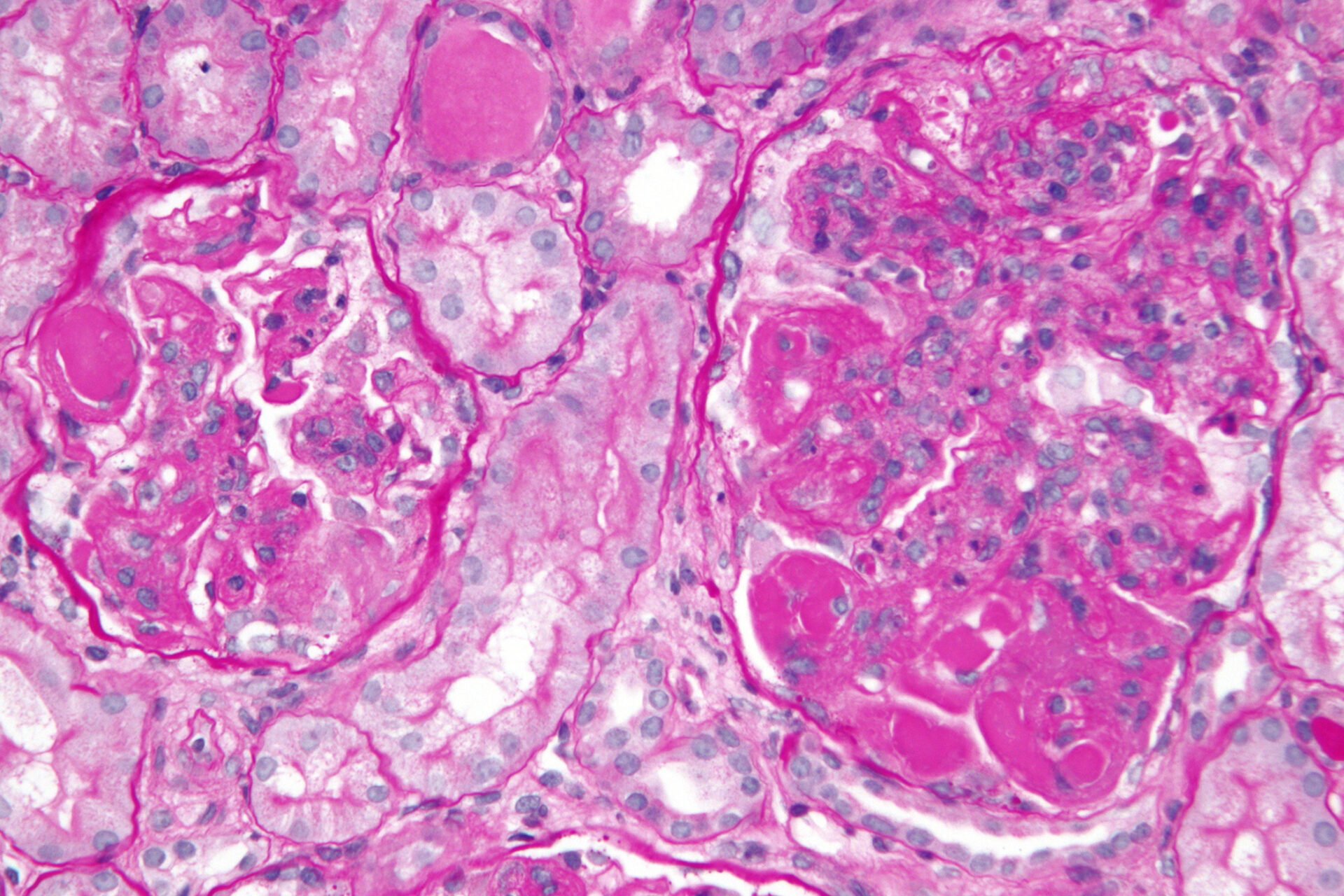
Cover picture: Very high magnification micrograph of diffuse proliferative lupus nephritis, class IV. PAS stain. ©Nephron, wikimedia