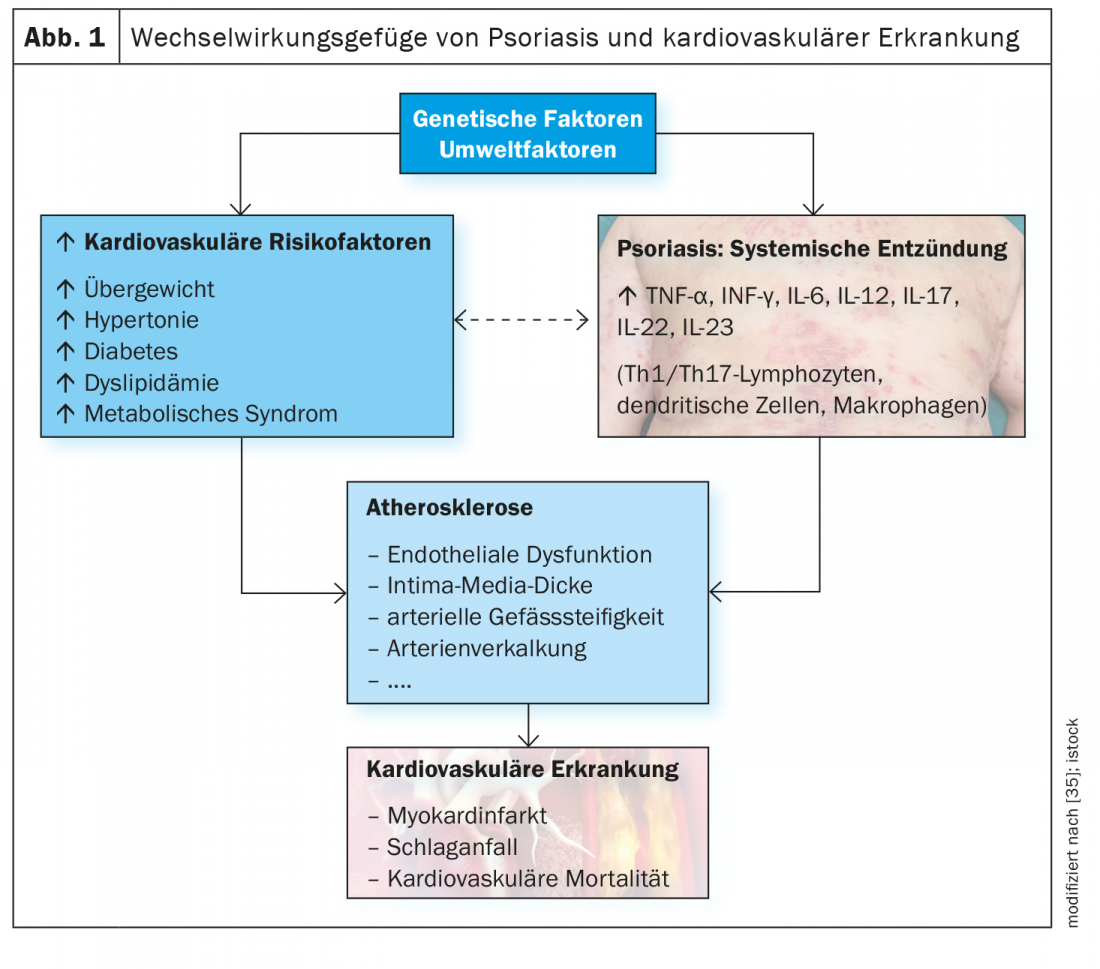
The IL23/Th17 axis plays an important role in the pathophysiology of psoriasis. A growing body of evidence suggests that inflammatory processes are involved in many cardiovascular and metabolic diseases associated with psoriasis. And there is evidence that upregulation of the IL23/Th17 pathway plays an important role in cardiometabolic comorbidities in psoriasis.
Cardiovascular and metabolic diseases are among the most common comorbidities of moderate to severe plaque psoriasis and contribute to an increased risk of mortality [1]. According to epidemiological data, cardiovascular disease (CVD) is the most common or second leading cause of death in psoriasis patients [2–4]. Especially in patients with severe psoriasis, the risk of cardiovascular events is considerably increased compared to the general population (myocardial infarction: RR 1.70-3.04; stroke: RR 1.38-1.59; cardiovascular mortality: 1.37-1.39) [5–8]. In addition to normotensive lipid profiles and oxidative stress, inflammatory processes are thought to be involved in the development of atherosclerotic changes and an associated increase in cardiovascular risk [9,10]. There is increasing evidence that chronic inflammation and immune dysregulation play an important role and that the IL23/Th17 pathway, among others, is involved.
Coronary heart disease
The risk of myocardial infarction as a manifestation of coronary heart disease (CHD) is increased by up to three times compared to non-psoriatics [11–13]. Genetic polymorphisms of IL23-R appear to be associated with the risk and severity of atherosclerosis [14,15]. Several studies show associations between IL23 and myocardial infarction. Yan et al. were able to show that IL23 increases sharply within three days after myocardial infarction, whereas IL23-R and IL17-A are upregulated for up to seven days and IL17-R for up to 14 days. Based on animal studies (mouse model), IL23 of macrophages and neutrophils is thought to serve as an upstream regulator of IL17-A and promote the production of γδT cells. IL17-A probably drives infiltration by neutrophils as well as fibrosis in myocardial tissue. Upregulation of IL23 was associated with higher infarct size, higher levels of typical biomarkers of myocardial injury (LDH and creatine kinase), and pro-inflammatory responses (increases in IL17A, IL6, and TNF-α) and pro-apoptotic effects. By activating JAK2/STAT3, IL23 induces the release of IL17-A, which ultimately enhances the inflammatory response and myocardial damage [16,17]. It was also found that neutralization of IL23 by anti-IL23p19 antibodies resulted in reduced IL17-A levels and a reduction in ischemic impairment and reperfusion injury [16,18].
Cerebrovascular disease
Inflammatory processes are considered to play an important role within the complex pathophysiology of ischemic stroke, especially for the exacerbation of brain injury. The occurrence of ischemia is accompanied by activation of microglia, resulting in the secretion of proinflammatory cytokines (especially IL23 and IL12) and neuroprotective mediators (IL10) [19]. Among the cells infiltrating the brain, macrophages are mainly involved in early phases of infarcts, whereas neutrophils and lymphocytes are involved in later phases [20]. IL23 secreted by macrophages and dendritic cells promotes the proliferation of Th17 and γδT cells and the production of IL17, which contributes to brain injury after stroke [21,22]. In human experimental studies, increased IL23 levels accompanied by an increased proportion of IL17-A-producing cells, as well as an increase in IL17-A levels and other cytokines, have been demonstrated compared with a control group at various time points after stroke [15]. In addition, a positive correlation was found between IL23 levels and lesion volume [15]. That an increase in pro-inflammatory mediators occurred concomitantly with a reduction in TReg cells (regulatory T cells) and IL10 levels supports the hypothesis that pro- and anti-inflammatory imbalance is a mechanism involved in stroke and brain injury [24].
The effect of blocking IL23/IL17 has been the subject of several studies. Animals with IL23 deficiency had significantly lower levels of γδT cells and consequently lower IL17 secretion and reduced infarct size [23]. Specific suppression of the IL23p19 subunit resulted in lower levels of the pro-inflammatory cytokines IL23 and IL17, accompanied by upregulation of the transcription factor FoxP3 (“forkhead box protein P3”) expressed by the TReg cell. Blockade of the p19 subunit was associated with a reduction in infarcts and neurological dysfunction, among other outcomes [21].
Peripheral arterial occlusive disease
Peripheral arterial disease (pAVD) of the lower limbs is a common clinical presentation in adults, often due to atherosclerosis. According to empirical data, the risk of pAVK is 98% higher in psoriasis patients compared to a control group (OR: 1.98; 95% CI: 1.32-2.82) [26] (Fig. 1). While there is a rather small evidence base regarding the role of inflammation in pAVD, a case-control study suggests a possible involvement of IL23 by showing that IL23 levels of pAVD patients were significantly higher compared to a control group [27].
Hypertension
Epidemiological studies show that the prevalence of hypertension is increased in psoriasis patients (OR: 1.58; 95% CI: 1.42-1.76) and has a positive correlation with psoriasis severity [1]. Moreover, psoriasis patients have an increased vulnerability to develop difficult-to-control hypertension, meaning they are 16.5 to 19.9 times more likely to require treatment with three or four medications compared to hypertensive patients without psoriasis [28]. That inflammatory processes play an important role in the pathogenesis of hypertension is well known, and it is also known that activated immune cells are a critical factor within this structure [29–31]. Notably, findings of involvement of the IL23/IL17 pathway have also been reported in this context. T cells and macrophages accumulate in the kidney and perivascular areas. Dendritic cells activate T cells, which promote Th17 differentiation by secreting IL6, TNF-α, and IL23, among others. Production of IL17-A stimulated by Th17 activation appears to be a critical factor in vascular dysfunction and maintenance of hypertension [31–33]. Because a dysbalance of Th17 and TReg is a pathophysiologically relevant factor for cardiovascular disease, Liu et al. investigated whether the use of anti-hypertensive drugs has an effect on this pathway [34]. They found that patients treated with a combination of telmisartan and rosuvastatin had a synergistic reduction in serum pro-inflammatory factors, including IL23, Th17 cells, and IL17-A, and an increase in anti-inflammatory factors, including TReg, FoxP3, and IL10 [34].
|
Summary The IL23/Th17 axis plays an important role in the pathophysiology of psoriasis. Up-regulation of this pathway, along with other inflammatory cytokines (e.g., TNF and type I IFN), contributes to the development of a chronic “pro-inflammatory state” in psoriasis. There is a growing body of evidence suggesting that inflammatory processes, through various mediators and pathways, are implicated in several cardiovascular diseases (e.g., coronary artery disease, hypertension) and chronic metabolic disorders (e.g., obesity, non-alcoholic fatty liver) clustered in psoriasis. Data suggest that upregulation of the IL23/Th17 pathway in combination with an unfavorable lifestyle is a possible explanation for many cardiometabolic comorbidities in psoriasis. With regard to therapeutic implications of these findings, there are still many open questions. For the hypothesis that highly specific monoclonal antibodies used for the treatment of psoriasis also have an effect on the course of comorbidities, the evidence base is still small. Further studies are needed to better understand these relationships. According to current knowledge, comorbidities in psoriasis patients should still be treated with the respective standard therapies. |
Literature:
- Takeshita J, et al: Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol 2017; 76: 377-390.
- Salahadeen E, et al: Nationwide population-based study of cause-specific death rates in patients with psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1002-1005.
- Svedbom A, et al: Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol 2015; 95: 809-815.
- Lee M-S, Yeh Y-C, Chang Y-T, Lai M-S: All-cause and cause-specific mortality in patients with psoriasis in Taiwan: a Nationwide Population-based Study. J Invest Dermatol 2017; 137: 1468-1473.
- Armstrong EJ, Harskamp CT, Armstrong AW: Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc 2013; 2: e000062.
- Samarasekera EJ, et al: Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol 2013; 133: 2340-2346.
- Raaby L, Ahlehoff O, de Thurah A: Psoriasis and cardiovascular events: updating the evidence. Arch Dermatol Res 2017; 309: 225-228.
- Mehta NN, et al: Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med 2011; 124: 775. e1-6.
- Zhou Q, Mrowietz U, Rostami-Yazdi M: Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 2009; 47: 891-905.
- Asha K, et al: Dyslipidaemia & oxidative stress in patients of psoriasis: emerging cardiovascular risk factors. Indian J Med Res 2017; 146: 708-713.
- Furue M, et al: Cardiovascular and metabolic diseases comorbid with psoriasis: beyond the skin. Intern Med Tokyo Jpn 2017; 56: 1613-1619.
- Hjuler KF, et al: Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med 2015; 128: 1325-1334.e2.
- Mahiques-Santos L, et al: Psoriasis and ischemic coronary artery disease. Actas Dermosifiliogr 2015; 106: 112-116.
- Zhang M, et al: Functional polymorphisms in interleukin-23 receptor and susceptibility to coronary artery disease. DNA Cell Biol 2014; 33: 891-897.
- Kave M, Shadman M, Alizadeh A, Samadi M: Analysis of the association between IL-23R rs11209026 polymorphism and incidence of atherosclerosis. Int J Immunogenet 2015; 42: 341-345.
- Hu X, Ma R, Lu J et al: IL-23 promotes myocardial I/R injury by increasing the inflammatory responses and oxidative stress reactions. Cell Physiol Biochem 2016; 38: 2163-2172.
- Liao Y, et.: Promoting effects of IL-23 on myocardial ischemia and reperfusion are associated with increased expression of IL-17A and upregulation of the JAK2-STAT3 signaling pathway. Mol Med Rep 2017; 16: 9309-9316.
- Zhu H, et al: Hmgb1-TLR4-IL-23-IL-17A axis promote ischemia-reperfusion injury in a cardiac transplantation model. Transplantation 2013; 95: 1448-1454.
- Zhao S-C, et al: Regulation of microglial activation in stroke. Acta Pharmacol Sin 2017; 38: 445-458.
- Ma S, et al: The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated ischemic stroke in mice. J Neuroimmunol 2013; 257: 28-35.
- Zheng Y, et al: Pivotal role of cerebral interleukin-23 during immunologic injury in delayed cerebral ischemia in mice. Neuroscience 2015; 290: 321-331.
- Brait VH, et al: Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab 2012; 32: 598-611.
- Gelderblom M, et al: IL-23 (interleukin-23)-producing conventional dendritic cells control the detrimental IL-17 (interleukin-17) response in stroke. Stroke 2018; 49: 155-164.
- Hu Y, Zheng Y, Wu Y, Ni B, Shi S: Imbalance between IL-17A-producing cells and regulatory T cells during ischemic stroke. Mediators Inflamm 2014; 2014: 813045.
- Jiang C, et al: Changes in the cellular immune system and circulating inflammatory markers of stroke patients. Oncotarget 2016; 8: 3553-3567.
- Prodanovich S, et al: Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 2009; 145: 700-703.
- David A, et al: Interleukin-23 serum levels in patients affected by peripheral arterial disease. Clin Biochem 2012; 45: 275-278.
- Armstrong AW, et al: Psoriasis and hypertension severity: results from a case-control study. PLoS ONE 2011; 6: e18227.
- Coffman TM: Under pressure: the search for the essential mechanisms of hypertension. Nat Med 2011; 17: 1402-1409.
- Kirabo A, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 2014; 124: 4642-4656.
- Dixon KB, Davies SS, Kirabo A: Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol 2017; 312: H368-H374.
- Loperena R, et al: Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res 2018; 114: 1547-1563.
- Madhur MS, et al: Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertens Dallas Tex 1979; 2010; 55: 500-507.
- Liu Z, et al: Treatment with telmisartan/rosuvastatin combination has a beneficial synergistic effect on ameliorating Th17/Treg functional imbalance in hypertensive patients with carotid atherosclerosis. Atherosclerosis 2014; 233: 291-299.
- Torres T, Bettencourt N: Psoriasis: The visible killer. Rev Port Cardio 2014; 33 : 95-99.
Further reading:
- Egeberg A, et al: The role of the interleukin-23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis. JEADV 2020; 34(8): 1695-1706, https://onlinelibrary.wiley.com/doi/10.1111/jdv.16273
DERMATOLOGY PRACTICE 2020; 30(6): 30-32