Often, cutaneous lymphomas receive little attention. And yet, a lot has happened in recent years. Thus, innovative treatment options are also emerging for these rare conditions through new therapeutic options such as antibody-drug conjugates, immunotherapy, and oncolytic viruses. Increasingly precise genetic and immunobiological characterization also enables increasingly differentiated diagnostics.
Often, cutaneous lymphomas receive little attention. And yet, a lot has happened in recent years. This was made clear by various experts from Zurich, Leiden and Birmingham at this year’s EADO Congress. Thus, innovative treatment options are also emerging for these rare conditions through new therapeutic options such as antibody-drug conjugates, immunotherapy, and oncolytic viruses. Increasingly accurate genetic and immunobiological characterization also allows for increasingly differentiated diagnosis, both for primary cutaneous B-cell and T-cell lymphomas.
Primary cutaneous B-cell lymphomas: of oncolytic viruses and immune checkpoints.
While cutaneous lymphomas tend to be thought of as T-cell neoplasms, about one in five primary cutaneous lymphomas is a B-cell lymphoma. Three subtypes are distinguished: primary cutaneous marginal zone lymphoma, primary cutaneous follicular lymphoma, and primary cutaneous large B-cell lymphoma. For all entities, Egle Ramelyte, M.D., of the University Hospital of Zurich, said new therapeutic opportunities may emerge in the near future, for example, through the targeted use of oncolytic viruses. This has already been approved for the treatment of malignant melanoma since 2015 and has recently been tested in cutaneous B-cell neoplasms [1]. By intralesional injection of the genetically modified herpes simplex 1 virus talimogene laherparepvec (T-VEC). promising results were achieved.
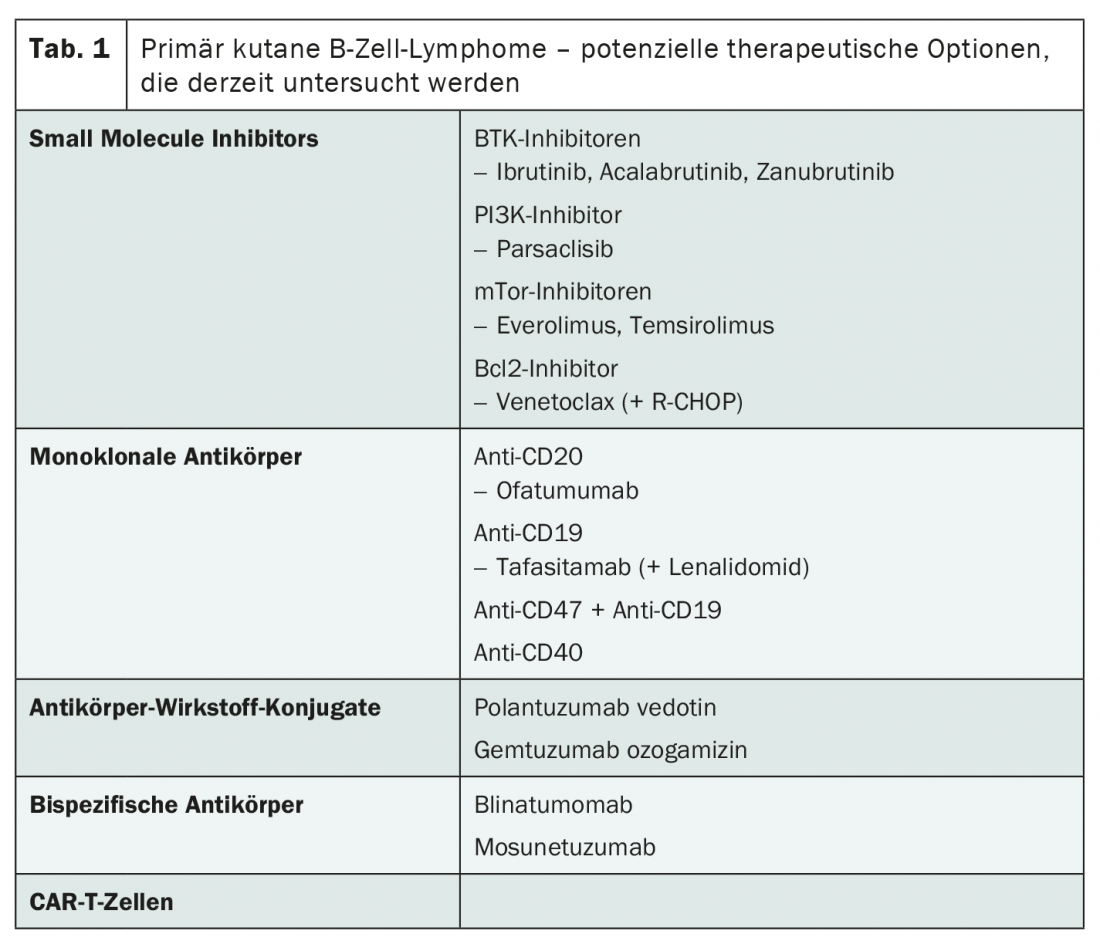
The current research landscape also potentially has a lot to offer for the therapy of advanced stages. While most compounds are being studied in nodal diffuse large B-cell lymphoma (DLBCL), the study results are likely to be relevant to primary cutaneous B-cell lymphomas as well , Ramelyte says. Thus, there may soon be alternatives to radiotherapy, rituximab and chemotherapy using R-CHOP. In addition to small molecule inhibitors and monoclonal antibodies, antibody-drug conjugates, bispecific antibodies and CAR-T cells are particularly worthy of consideration (Tab. 1) . The latter are already approved in Switzerland for the treatment of relapsed or refractory DLBCL – with great success.
In contrast to other immunotherapeutic options such as bispecific antibodies, which bind the tumor cell on the one hand and the body’s own T cells on the other and thus lead to the fight against the malignancy, the expert sees lower chances of success for checkpoint inhibitors in the therapy of cutaneous B-cell neoplasms. Although these have permanently changed the therapeutic landscape of solid tumors, they have had little success in hematological neoplasms apart from Hodgkin’s lymphoma. The response rate in DLBCL to anti-PD1/anti-CTLA4 combination therapy is less than 30%, Ramelyte said. Nevertheless, various combination treatments with checkpoint inhibitors are currently under investigation. In addition, LAG3, a potential novel immune checkpoint expressed by over 70% of DLBCL, is being explored. LAG3 is likely to be associated with a worse prognosis, independent of the International Prognostic Index , according to a recent study [2]. This new checkpoint could thus acquire both diagnostic and therapeutic relevance.
Also of diagnostic importance may be findings in the areas of tumor genetics and immunohistochemistry. Currently, the value of the two immunohistochemical markers IRTA1 and MNDA, which have been mainly used for the diagnosis of extracutaneous MALT lymphomas, is being tested [3]. It has also been shown in recent years that most cutaneous DLBCL – and nearly 80% – carry a mutation in the MYD88 gene. This leads to activation of the transcription factor NF-κB. In those tumors whose MYD88 gene is not mutated, another mutation that activates NF-κB can be detected in most cases [4].
Cutaneous T-cell lymphomas: of flow cytometry and genetic variance.
According to current research, the genetic landscape of cutaneous T-cell lymphomas is much more heterogeneous than in cutaneous DLBCL. This could be analyzed in more detail by using next generation sequencing. Overall, large genetic differences were seen both between affected individuals but also within the same tumor. In particular, according to Prof. Maarten Vermeer, MD, Department Head of the Department of Dermatology at Leiden University Hospital in the Netherlands, genomic integrity is affected by P53 mutations, various signaling pathways such as the NF- κB and JAK/STAT signaling pathways, and the epigenetic landscape is affected by genomic aberrations. Despite the diverse picture, the characteristic changes in DNA methylation and histone modification could be of diagnostic value and potentially serve as markers in the future – with names like CMTM2, PROM1 or GNMT [5,6]. This is because there is currently a lack of reliable diagnostic tools for the unequivocal identification of cutaneous T-cell lymphomas. And that is not the only drawback of the large genetic variance: Vermeer warns of potential resistance to targeted therapies through the selection of tumor cell subpopulations.
Even if there is still some uncertainty in diagnostics today, especially in the field of blood diagnostics, important progress has been made since the era of morphological identification of circulating tumor cells. The correct classification of such circulating malignant cells is important in that it is crucial for staging and thus prognosis and therapy. The widespread adoption of flow cytometry for staging has standardized and improved this in recent years, although standardization of the procedure and identification of appropriate markers remain challenges [7].
Prognosis and therapy in transition
There are also still uncertainties regarding prognostic factors of cutaneous T-cell lymphomas. However, new prognostic markers such as total mutation burden and tumor clone frequency (TCF) in the lesion have emerged over the past few years. A TCF greater than 25% was a stronger predictor of progression than all other established prognostic factors in the corresponding study [8]. A prerequisite for the determination of TCF is the identification of the T-cell clone, which can now be performed by high-throughput sequencing with significantly higher sensitivity than traditional PCR (polymerase chain reaction) . However, this method is expensive and is only used in a few centers so far. To finally provide clarity in the field of prognostics, the PROCLIPI (Prospective Cutaneous Lymphoma International Prognostic Index Study) study was launched in 2015, collecting continuously defined data and materials for a purpose-built biobank. A study duration of 10 years is planned, and 1991 patients from 19 countries have already been recruited.
In addition to exploring prognostic factors, evaluation of current therapies is also a goal of the PROCLIPI trial. Since 2017, three new compounds have been approved in the European Union for the treatment of cutaneous T-cell lymphoma: Brentuximab vedotin, Mogamulizumab and Chlormethine Gel. The former is also available in Switzerland. While chlormethine gel can be used for topical therapy in all stages of mycosis fungoides, the antibody-drug conjugate brentuximab vedotin and the monoclonal antibody mogamulizumab are used for the treatment of recurrent cutaneous T-cell lymphomas.
Even with these developments, allogeneic stem cell transplantation, performed since 1980, remains the only curative option. Improvements in methods for T-cell depletion, less intensive regimens, and new insights into the management of graft-versus-host disease (GvHD) have also made it more tolerable. A review published in 2019 reported a 1-year mortality of 15% with a recurrence rate of 50% [9]. In this context, Prof. Dr. med. Julia Scarisbrick from the University Hospital in Birmingham, England, emphasized that early use of allogeneic stem cell transplantation should be increasingly evaluated. Finally, a complete response to any therapy is rare in advanced stages, and transplantation to patients with residual disease has not been shown to be effective. She therefore advocated evaluation of stem cell transplantation in the first remission.
Source: Symposium SY13 “Cutaneous lymphomas: what is new?” by Prof. Dr. med. Maarten Vermeer (Leids Universitair Medisch Centrum), Dr. med. Egle Ramelyte (University Hospital Zurich) and Prof. Dr. med. Julia Scarisbrick (University Hospital Birmingham), April 15, 2021, 10th World Congress of Melanoma/17. EADO Congress, the congress took place virtually.
Literature:
- Ramelyte E, et al: Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell. 2021; 39(3): 394-406. e4.
- Keane C, et al: LAG3: a novel immune checkpoint expressed by multiple lymphocyte subsets in diffuse large B-cell lymphoma. Blood Adv. 2020; 4(7): 1367-77.
- Wang Z, Cook JR: IRTA1 and MNDA Expression in Marginal Zone Lymphoma: Utility in Differential Diagnosis and Implications for Classification. Am J Clin Pathol. 2019; 151(3): 337-343.
- Zhou XA, et al: Genomic Analyses Identify Recurrent Alterations in Immune Evasion Genes in Diffuse Large B-Cell Lymphoma, Leg Type. J Invest Dermatol. 2018; 138(11): 2365-2376.
- Qu K, et al: Chromatin Accessibility Landscape of Cutaneous T Cell Lymphoma and Dynamic Response to HDAC Inhibitors. Cancer Cell. 2017; 32(1): 27-41. e4.
- van Doorn R, et al: Epigenomic Analysis of Sézary Syndrome Defines Patterns of Aberrant DNA Methylation and Identifies Diagnostic Markers. J Invest Dermatol. 2016; 136(9): 1876-1884.
- Scarisbrick JJ, et al: Developments in the understanding of blood involvement and stage in mycosis fungoides/Sezary syndrome. Eur J Cancer. 2018; 101: 278-280.
- de Masson A, et al: High-throughput sequencing of the T cell receptor β gene identifies aggressive early-stage mycosis fungoides. Sci Transl Med. 2018; 10(440).
- Johnson WT, et al: Allogeneic hematopoietic stem cell transplantation in advanced stage mycosis fungoides and Sézary syndrome: a concise review. Chin Clin Oncol. 2019; 8(1): 12.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(3): 28-29 (published 6/15-21, ahead of print).