Ruptured abdominal aortic aneurysm (rBAA) can be treated either openly surgically or minimally invasively, i.e. endovascularly. Enthusiastic success stories suggest that the newer endovascular technique has finally made a breakthrough in reducing stagnant mortality. However, a more detailed analysis shows that similar good results can be achieved with both techniques. Thus, the prognosis of rBAA seems to be determined mainly by factors related to the patient’s constitution and peri-operative management. Two key advantages of open aortic replacement are that it can be performed anywhere a skilled team is available and does not rely on a specialized infrastructure. In addition, it can be used regardless of anatomical conditions. Therefore, open aortic replacement should continue to be the reference standard for rBAA until the superiority of a particular technique is demonstrated in a clean scientific manner without prejudice or expertise is available.
Abdominal aortic aneurysm (BAA) is a disease of the aging male and typically develops at the base of atherosclerosis. The increase in size is usually gradual (3 mm/y on average), which is why most BAA remain asymptomatic for a long time, although the wall stress increases with increasing diameter and the vessel wall thins out. The feared consequence is rupture, which is associated with high mortality. Epidemiological studies suggest that the prevalence of BAA has been slowly decreasing since 1999 (currently about 2.2% in men over 65 years of age ) [1]. In parallel, the incidence of rupture is decreasing, mainly because fewer and fewer people smoke and older and older patients are undergoing elective surgery before the BAA ruptures [2]. In contrast, peri-operative mortality in the event of rupture has barely decreased (still averaging 30-50%) [3, 4], which is surprising given other advances in peri-operative patient care.
Open and endovascular aortic replacement -two treatment alternatives.
Intact BAA are treated virtually everywhere today in one of two ways: Open aortic replacement using a plastic prosthesis (Dacron or ePTFE) has been performed for about 60 years and is a proven technique with excellent long-term durability. Endovascular aortic replacement (EVAR) using stent-graft prosthesis has been available as a minimally invasive alternative for about 15 years.
The first randomized trials have consistently shown that EVAR can reduce peri-operative mortality by a factor of up to 3 compared with open surgery [5–7]. However, none of these studies showed a long-term survival benefit, and by their nature, the study results apply only to aneurysms that have favorable morphology on imaging. Despite these limitations, the elective results suggest that a peri-operative survival benefit of EVAR may be particularly important in ruptured BAA. And indeed, this assumption seems to be confirmed by meta-analyses of (mainly uncontrolled) case series suggesting a reduction in mortality from an average of 40% to 20% [8]. However, first, the patients in these series were selected differently and, second, randomized comparisons (which, by the way, again studied only EVAR-eligible patients!) have so far shown no mortality differences between open and endovascular aortic replacement. In the first, very small, randomized trial, mortality was identical at 53% [9]; in the larger AJAX (acute endovascular treatment to improve outcome of ruptured aortoiliac aneurysms) trial, there also appeared to be no statistically significant difference [10]. However, the publication of this study is still pending.
Elective EVAR differs fundamentally from emergency EVAR for rupture in at least three respects. First, the latter, by its very nature, cannot be planned in advance. This means permanent reserve services in terms of expertise, infrastructure and a stent graft assortment. However, since only 50-60% of ruptured BAA are anatomically suitable for EVAR [8], open surgical back-up still cannot be saved. Second, suprarenal aortic ballooning is sometimes required for acute hemorrhage control, which can lead to visceral ischemia, the deletive consequences of which easily go unnoticed. Third, the aortic rupture site prevents the compartmentalization of the aneurysm sac from causing the rebleeding lumbar arteries to cause an abdominal compartment syndrome [11]. Against this background, doubts about a causal relationship between the (technical) treatment method and the peri-operative mortality risk are appropriate.
The purpose of this article is to illustrate the importance of other (independent) treatment strategies that have received little attention to date. It is important that the concepts are independent of a complex technical infrastructure, so that they can be implemented anywhere, at least in theory.
Key factors of successful rBAA treatment
The prognostic significance of various treatment aspects was recently investigated in a homogeneous consecutive patient series from a single center that guarantees care for approximately 1.5 million residents [8, 12, 13]. The cohort was predestined for such analyses in that virtually all patients received the same treatment (namely, open surgery). This mitigates the confounding influence of method choice and patient selection. Specifically, all patients treated for rBAA between January 2001 and December 2012 were included in a prospective data registry [8, 12, 13].
All aneurysms in which blood was detected by imaging or found intraoperatively outside the vessel wall were analyzed unselected (ie, true aneurysms, pseudoaneurysms, and mycotic aneurysms, each located juxta- or infrarenally and/or iliac). Patients in the first decade (2001-2010) have been studied in more detail in several papers(Fig. 1) [8, 12, 13]. Almost all (97%) underwent open surgery, and survivors were followed for a median period of 3.2 years (interquartile range [iqr] 1.1-5.4 years).

Fig. 1: The chart shows the flow of patients from January 2001 to the end of 2012. Using data from the decade 2001 to the end of 2010 (n=248 patients), separate analyses were performed to estimate the influence of various preoperative factors on survival of patients with ruptured BAA.
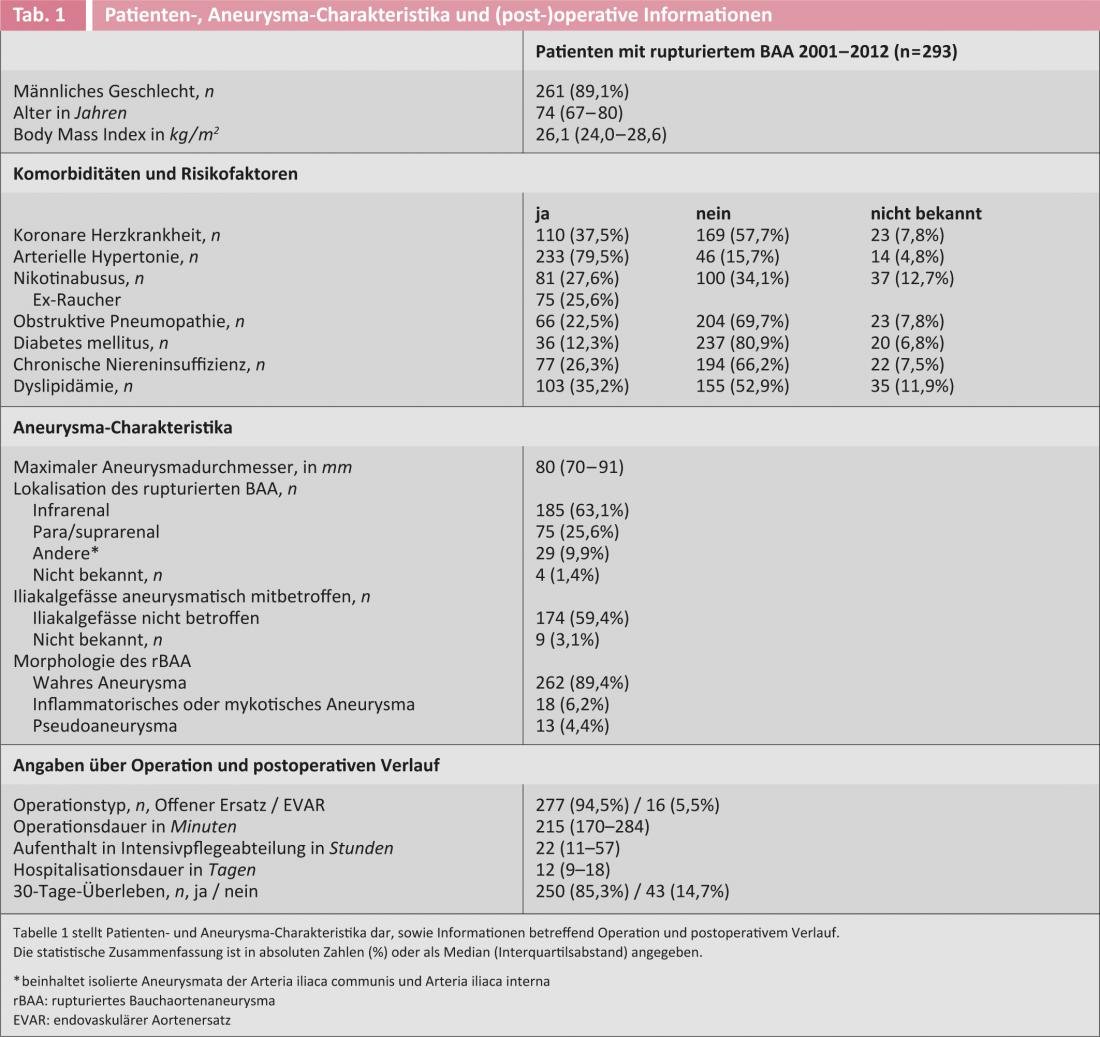
Peri-operative mortality (defined as 30-day mortality; this information was known in all patients) in the entire series (n=293) was 14.7%. Only 10% of patients had not been offered the treatment for a variety of reasons. Thus, the results of this unselected patient series are at least as good as in the best published EVAR series [14], especially considering that the latter report only carefully selected patients. Among the 248 patients in the first decade, detailed below, 30-day mortality was in a comparable range at 15.3%. Patient- and aneurysm-associated characteristics as well as surgical and peri-operative information are recorded in Table 1.
“Favorable aortic anatomy” as an explanation?
Until a causal effect is demonstrated in randomized experiments, the survival benefit of EVAR observed in meta-analyses can be explained in two basic ways:
- Either (a) EVAR is indeed conceptually superior in rBAA (=causal effect),
- or else (b) in patients who qualify for emergency EVAR, there is an independent survival advantage (=selection bias).
If variant (b) were correct, EVAR-suitable patients should also perform better after open aortic replacement than EVAR-unsuitable patients. In the above rBAA cohort, all cases were classified as “EVAR-eligible,” “EVAR-uneligible,” or “not assessable” by two independent investigators according to predefined criteria [8]. This resulted in the following ordinal categories and one additional category: (1) “EVAR-suitable” (when both investigators agreed), (2) “questionably suitable” (when they disagreed), (3) “EVAR-unsuitable” (when both agreed), and (4) “uncategorizable.” Consistent with previous reports, (of these openly operated patients!) 54% (n=133) were classified as EVAR-eligible (28% “EVAR-eligible” and 26% “questionably eligible”) and 40% (n=100) as “EVAR-uneligible.” The remaining 6% (n=15) could not be categorized, for example, because of missing CTs.
The 30-day mortality followed the ordinal categories in an impressive manner [8]: “EVAR-eligible” patients had a risk of death of 4%, “questionably eligible” of 16% (odds ratio [OR] 4.73 [95% Konfidenzintervall [KI] 1.13-19.77; p=0.033]) and “EVAR-uneligible” of 24% (OR 8.03 [95% CI 2.06-31, 34; p=0.003]) as reference category. Results were corrected for potential confounders and remained unchanged in sensitivity analyses, for example, when uncategorizable patients were assigned to each category in turn. That is why the positive effect of favorable anatomy seems to be indeed independent of the treatment technique and other influencing factors and very robust. Thus, anatomy explains at least part of the observed difference between EVAR (selection of anatomically favorable patients) and open aortic replacement (depending on the center, patients tend to be unsuitable for EVAR because anatomically favorable patients are treated by EVAR).
Surgical “high risk” patients
Traditional surgical risk stratification models such as the Hardmann score suggest that elderly patients with rBAA (i.e., older than 75-80 years, for example) carry a prohibitively high surgical risk and therefore should be rejected for emergency open aortic replacement. EVAR could theoretically offer a decisive advantage in these patients. However, we know from the (elective!) EVAR study 2 that EVAR does not confer a benefit in surgically inoperable patients [15]. In the rBAA cohort studied, the question of whether advanced age per se negatively affects peri-operative mortality to such an extent that it is justified as a reason for rejection was addressed.
24% of patients (n=60) were older than 80 years. As expected, 30-day mortality in bivariate analysis was higher in these patients (26.7% vs. 11.7%; p=0.007). However, in multivariate analyses that corrected the result for confounders, advanced age lost its statistically independent disadvantage (OR to die peri-operatively was 2.1 [95% CI 0.9-5.2; p=0.099]). Indeed, the negative age effect was primarily based on the fact that the older patients were also more likely to have coronary artery disease, which in turn was associated with higher mortality. This is also consistent with the fact that within the elderly patient group, a shock index >1 (in terms of limited cardiac reserve) was the most important predictor of increased mortality [12]. Interestingly, patients who survived the first 30 days had the same survival prognosis as the age-, sex-, and calendar year-matched overall population-regardless of age at rBAA surgery [12].
Thus, advanced age alone is not a valid exclusion criterion from emergency aortic replacement. In otherwise healthy elderly patients, the results of open surgery may well be equivalent to EVAR. However, open aortic replacement does not provide a benefit in elderly patients with coronary artery disease who are already assigned in shock. That EVAR could lead to better results here is hypothetically possible, but has not yet been shown.
The peri-operative volume management
The most important prognostic factor-aside from rapid emergency intervention-may be volume and hemodynamic management between diagnosis and surgical hemostasis. It is known from trauma studies and animal studies that volume restriction and permissive arterial hypotension until surgical hemostasis save more lives in hemorrhage shock than volume-assisted “stabilization” of the circulation [13]. Tolerance of the shock state, especially in rBAA patients who are often more polymorbid and older than trauma patients, seems counter-intuitive at first glance but is compelling in theory. The lower bleeding pressure and decreased dilutional coagulopathy promote coagulation and tamponade of the retroperitoneum, which reduces blood loss. In addition, volume restriction mitigates the risk of hypothermia and acid-base imbalance.
Interestingly, based on such considerations, the concept of “volume restriction until surgical hemostasis” is also repeatedly recommended in rBAA patients but has never been systematically studied. Therefore, this concept was explored in the cohort studied. Information on the amount of volume delivered preoperatively and rate of volume delivery (in l/h) before clamping the aorta was collected from the transfer and anesthesia logs and correlated with 30-day mortality after emergency open aortic replacement after correction for possible confounders [13].
Both volume rate and absolute volume administered had an independent and statistically significant negative effect on the risk of death within the first 30 days. Specifically, each additional liter of volume per hour increased the risk of mortality by 1.57-fold (95% CI 1.1-2.3; p=0.026). This was independent of patient condition, mean blood pressure, and other patient factors or therapeutic measures. The effect was mainly determined by the volume rate rather than the total volume [13].
Conversely, this means that consistent volume restriction through the entire rescue chain, i.e., from diagnosis to emergency intervention, is a prognostically important and efficient concept in patients with rBAA that helps to ensure their survival. Consequently, all actors should be trained in this sense. The control of volume substitution in the awake patient depends primarily on the state of consciousness. In opacifying patients, systolic blood pressure should be supported but not raised above 70-80 mmHg for prolonged periods. Although these analyses apply to patients undergoing open surgery, there is no reason not to assume a similar beneficial effect of volume restriction in EVAR patients.
Conclusion
Factors such as (1) an efficient rescue chain with rapid diagnosis and expeditious transport to the treating hospital, (2) consistent volume restriction with permissive hypotension, (3) rapid and systematic radiologic workup, (4) prognostically meaningful and ethical risk selection, and (5) the constant presence of a competent and practiced treatment team save many more lives of patients with rBAA than a particular treatment technique or implant. For optimal results, the treatment team must do what it does best and what the infrastructure is suited for, keeping these factors in mind.
Regula von Allmen, MD
Prof. Dr. med. Jürg Schmidli
PD Matthias Widmer, MD
PD Florian Dick, MD
Literature:
- Svensjo S, et al: Circulation 2011; 124: 1118-1123.
- Anjum A, et al: The British journal of surgery 2012; 99: 637-645.
- Bown M.J, et al: The British journal of surgery 2002; 89: 714-730.
- Holt PJ, et al: The British journal of surgery 2010; 97: 496-503.
- Greenhalgh RM, et al: Lancet 2004; 364: 843-848.
- Blankensteijn JD, et al: The New England journal of medicine 2005; 352: 2398-2405.
- Lederle FA, et al: JAMA : the journal of the American Medical Association 2009; 302: 1535-1542.
- Dick F, et al: The British journal of surgery 2012; 99: 940-947.
- Hinchliffe RJ, et al: European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery 2006; 32: 506-513; discussion 14-15.
- Balm R.: Oral presentation at Charing Cross Symposium, London, 15th April 2012.
- Mayer D, et al: Journal of vascular surgery 2009; 50: 1-7.
- Opfermann P, et al: European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 2011; 42: 475-483.
- Dick F, et al: Journal of vascular surgery 2013.
- Veith FJ, et al. Ann Surg 2009; 250: 818-824.
- Greenhalgh RM, et al: The New England journal of medicine 2010; 362: 1872-1880.











