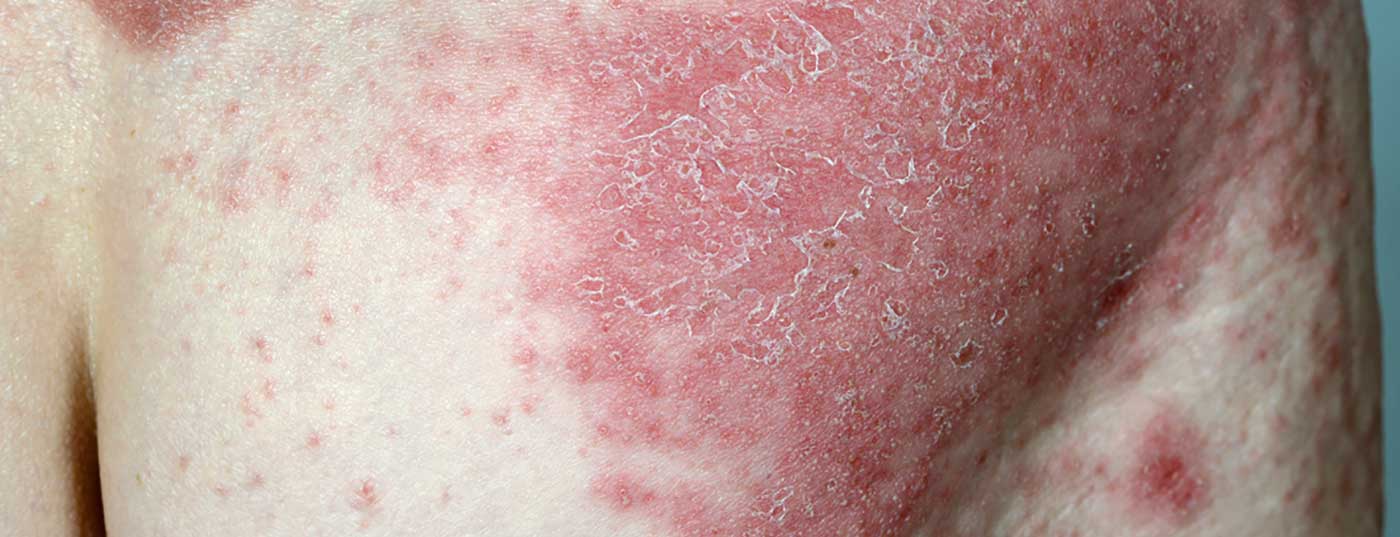
Continuous therapy with the anti-IL17 antibody ixekizumab has shown sustained long-term improvements in itch, skin pain, and health-related quality of life in patients with moderate to severe plaque psoriasis. Patients were also shown to benefit in terms of work-related productivity. This is from an analysis of data from the extension phases of the UNCOVER-1 and -2 trials with a follow-up phase through week 264.
Participants included in UNCOVER-1- and -2 were adult patients with moderate-to-severe psoriasis (BSA ≥10, sPGA ≥3, PASI ≥12) who were eligible for systemic therapy. In UNCOVER-1 (n=1296), study participants were randomized 1:1:1 to ixekizumab 80 mg every 2 weeks (q2w), ixekizumab 80 mg every 4 weeks (q4w), or placebo. In the UNCOVER-2 trial, participants (n=1224) were randomized 2:2:2:1 to ixekizumab q2w, ixekizumab q4w, etanercept 50 mg 2× weekly, or placebo. In both studies, patients treated with ixekizumab received an initial loading dose of 160 mg.
Included in the present analysis of long-term data were participants in the UNCOVER-1 and -2 trials who were randomized to ixekizumab every 2 weeks and received ixekizumab at intervals of 4 weeks during the maintenance phase (weeks 12-60) [1]. Another criterion was that a physician global assessment (PGA) score of 0 or 1 was achieved at week 12 and study participation continued until week 60 with inclusion in the subsequent extension phase. Patient characteristics and disease features of the long-term extension phase study population were comparable to the overall study population [2,3].
Improvements in various patient-reported outcomes (PROs).
Patient-reported outcomes (PROs) are subjective assessments of health-related aspects as an adjunct to standard clinical measures assessed by physicians. Patient-reported outcomes included in the present analysis were the numerical rating scale for pruritus (NRS-itch), skin pain VAS (visual anaolgic scale), and the dermatological quality of life index DLQI (score of 0 or 1). Also collected were mean change in the SF-36 (“Short Form health survey”) and in the physical and mental components of this health questionnaire (PCS and MCS, respectively).
Psoriasis-related appearance distress was operationalized by the PSAB (“Psoriasis Skin Appearance Bothersomeness”), and the WPAI (“Productivity and Activity Impairment Questionnaire”) was used as a measurement tool to capture subjectively perceived impairment in work-related productivity.
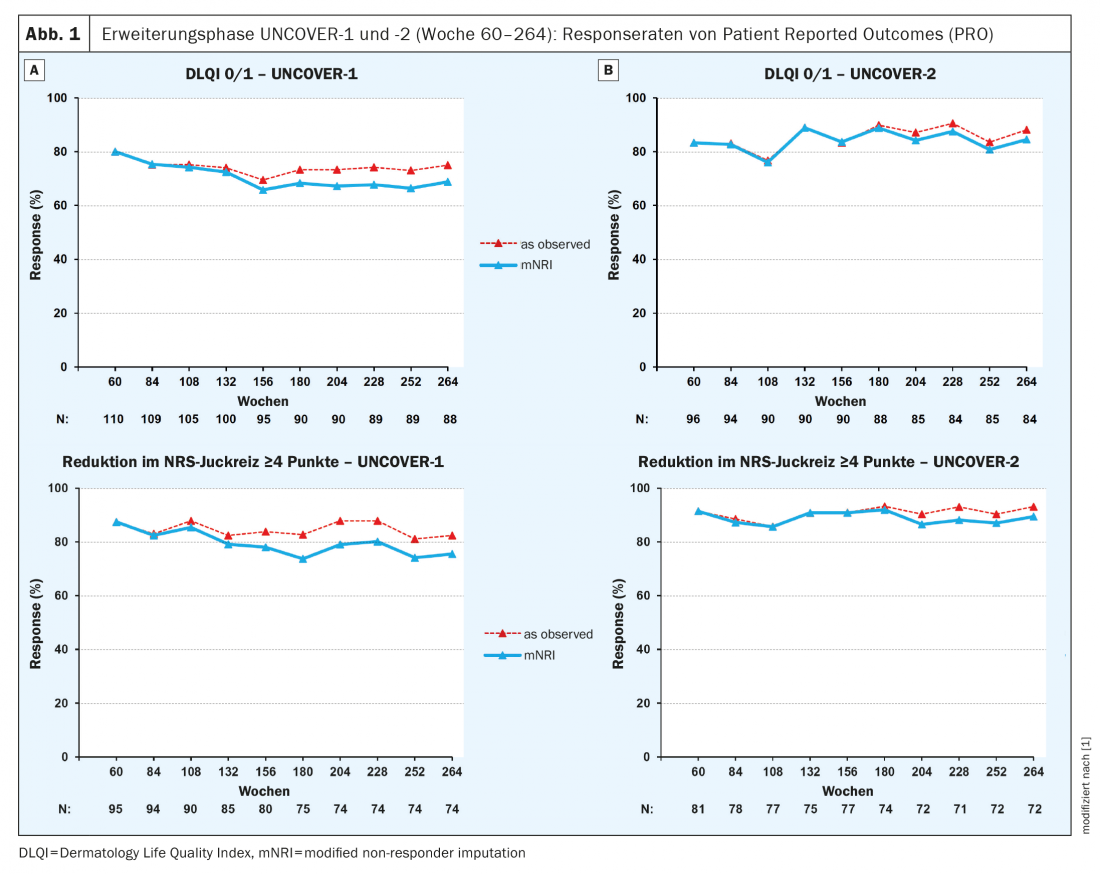
At week 264, in UNCOVER-1 and -2, a proportion of 82.4% and 93.1%, respectively, achieved a reduction in NRS itch ≥4 points (Fig. 1), an NRS score of 0 was exhibited by 51.7% and 58.5%, respectively, and the proportion of those with VAS skin pain of 0 was 59.3% and 63.1%, respectively. Regarding DLQI 0 or 1, the corresponding rates were 75.0% and 88.1%, respectively (Fig. 1). Changes from baseline to week 264 in other parameters were as follows: SF-36 MCS: 3.4 and 6.5, respectively; SF-36 PCS: 4.4 and 4.8, respectively; PSAB-21.3 and -22.0, respectively. There were improvements in item scores in WPAI psoriasis since baseline in both UNCOVER-1 and -2.

In summary, 264 weeks of treatment with ixekizumab resulted in sustained improvement in several PROs, including pruritus NRS and DLQI, in both the UNCOVER-1 and UNCOVER-2 trials.
Source: Eli Lilly
Literature:
- Gooderham MJ, et al: Effect of Ixekizumab on Patient Reported Outcomes and Quality of Life in Patients With Moderate-to-Severe Plaque Psoriasis: 5-Year Results from the UNCOVER-1 and -2 Studies. J Drugs Dermatol 2021; 20(4): 394-401.
- Gordon KB, et al: Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New Engl J Med 2016; 375: 345-356.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. New Engl J Med 2016; 375: 2102.
DERMATOLOGIE PRAXIS 2022; 32(1): 30-31