Increasing understanding of the pathogenetic relationships of renal cell carcinoma has contributed to the development of new drugs. These specifically interfere with the VEGF, PDGF and mTOR signaling pathways. In recent years, therapies have also been developed that target the immune system to fight tumors. Where are we at mRCC today? What do the current guidelines say?
Renal cell carcinoma is comparatively rare in Switzerland, with an incidence of about 950 cases and 1.8% of cancer deaths per year. Men are affected significantly more often than women (67.9% vs. 32.1%) and about one third of patients already have local or distant metastases at diagnosis [1]. Approximately 25% of patients with localized disease develop distant metastases after curatively designed nephrectomy [2].
Of the numerous subtypes, light cell renal cell carcinoma is the most common, accounting for 70-75% of cases, and is associated with inactivation of Von Hippel-Lindau (VHL) genes, leading to increased hypoxia-induced factor (HIF) activity and ultimately overexpression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [3,4]. The activity of HIF can also be enhanced by the Mammalian Target of Rapamycin (mTOR) signaling pathway [5]. Understanding these relationships has been instrumental in the development of new drugs that specifically target VEGF, PDGF, and mTOR signaling pathways (Fig. 1).
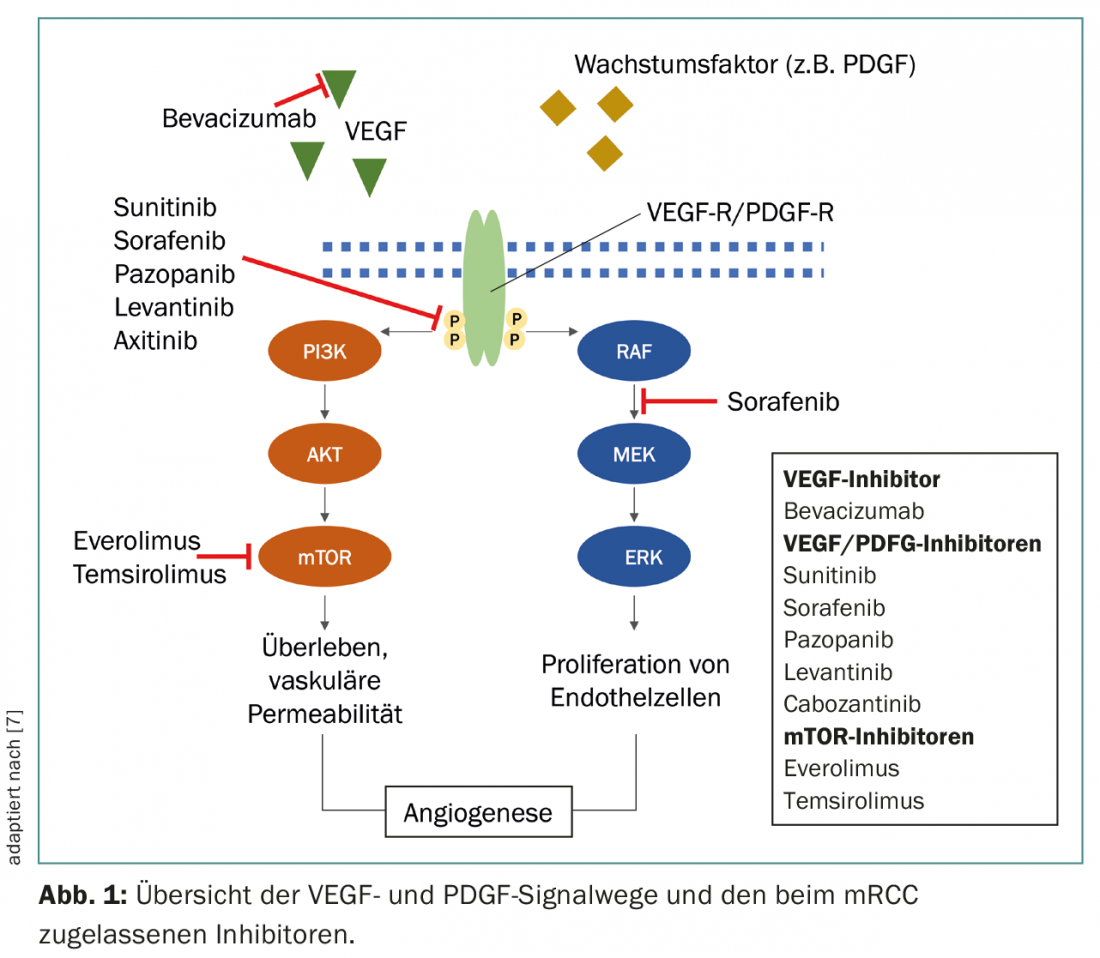
In recent years, therapies have also been developed that direct the immune system to fight the tumor. Expression of CTLA-4 and PD-1 by activated T cells leads to a reduction in T cell activity at different stages of the immune response. The anti-CTLA-4 monoclonal antibody (mAB) ipilimumab and the anti-PD-1 mAB nivolumab help induce an effective anti-tumor T-cell response by blocking these checkpoint receptors [6].
Risk group classification and guidelines
Prognostic models and the classification of patients into different risk groups form an important basis for therapy decisions. Memorial Sloan Kettering Cancer Center (MSKCC) criteria predate the establishment of targeted therapies [8]. Alternatively, the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) has defined factors as a basis for risk assessment [9,10]. The two regimens differ in only a few respects and divide patients into favorable, intermediate, and unfavorable risk groups (Table 1) . The treatment of mRCC has been in constant flux since the first approval of a targeted therapy (sunitinib, 2006). New guidelines from the European Association of Urology (EAU) were published in early 2018, which, based on the CheckMate-214 study, already list combination therapy with nivolumab/ipilimumab in intermediate/unfavorable risk patients [11]. In the study, the combination of nivolumab and ipilimumab compared with sunitinib achieved a 1.5-year survival rate of 75% versus 60% with sunitinib after a median follow-up of 25.2 months (HR 0.63, p<0.001). Median progression-free survival (PFS) was 11.6 months in the nivolumab/ipilimumab group and 8.4 months in the sunitinib group; however, this difference between treatment arms was not statistically significant (HR 0.82, p=0.03) [12]. The objective response rate was 42% versus 27% (p<0.001) and the rate of complete remissions was also higher with the immunotherapy combination (9%) than with sunitinib (1%). The combination therapy ipilimumab/nivolumab has recently been approved in Switzerland for the treatment of mRCC. Currently, the Swiss Association for Clinical Cancer Research (SAKK) is conducting a phase II trial with ipilimumab and nivolumab in the first or second line of therapy (SAKK 07/17).
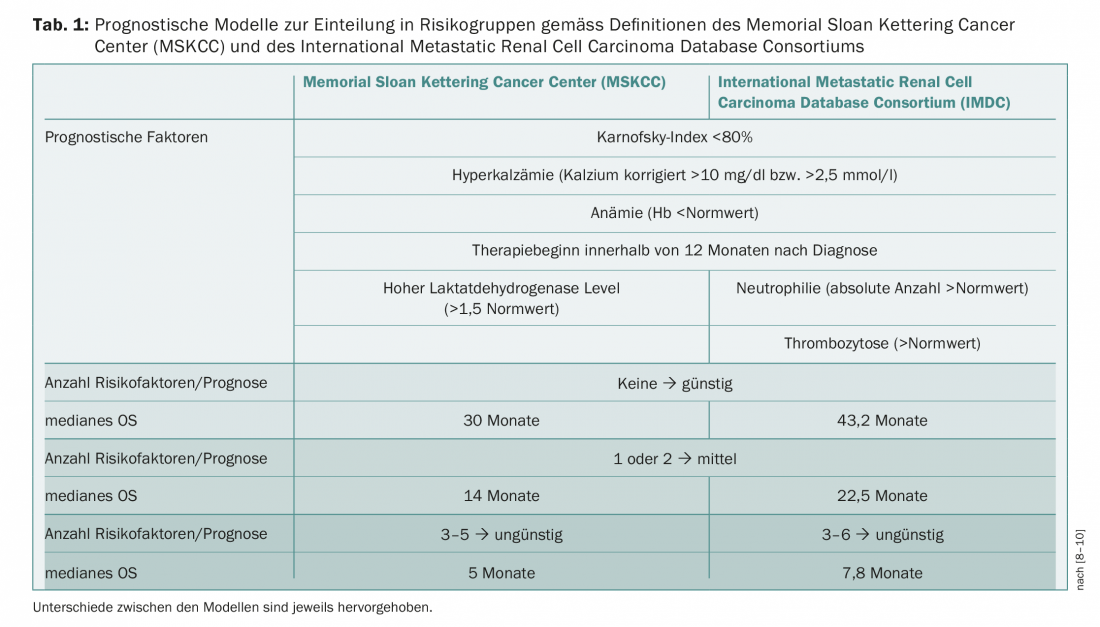
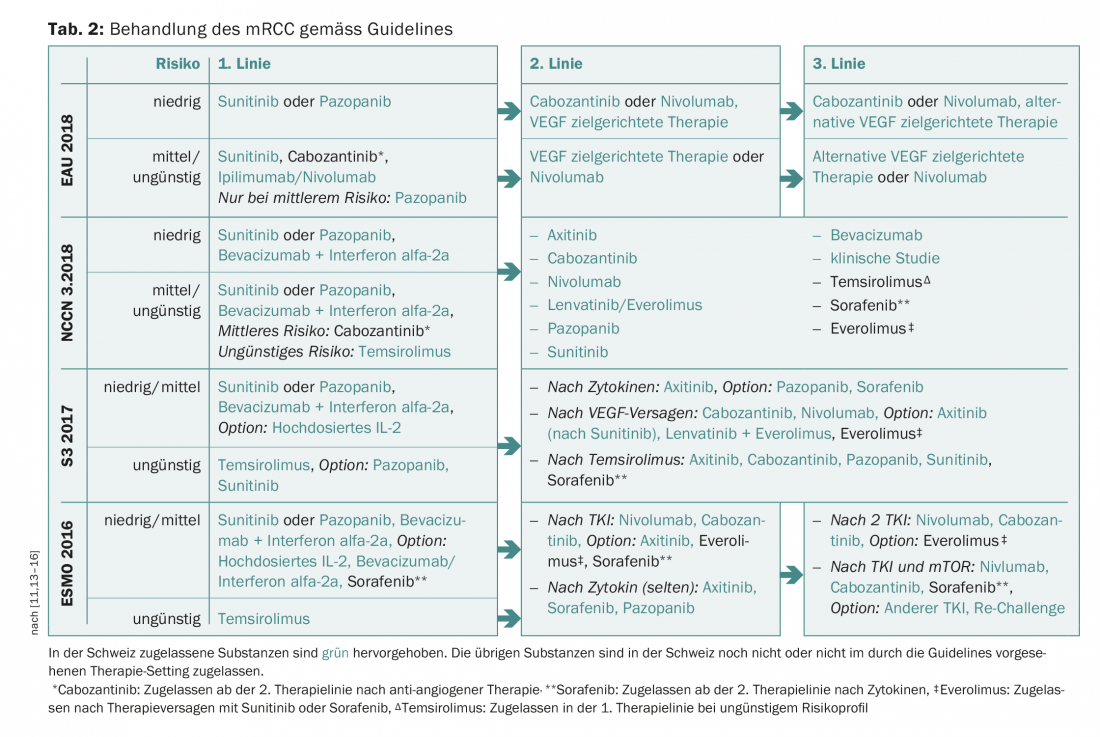
In the following, mainly the therapy management with the targeted, anti-angiogenic tyrosine kinase inhibitors (TKIs) and the mTOR inhibitors will be discussed. Table 2 shows an overview of the current guidelines with a focus on the substances approved in Switzerland.
Nephrectomy as standard of care in mRCC.
Nephrectomy has been an integral part of mRCC therapy for nearly 20 years, as it has been shown that surgery prior to interferon alfa (IFN-α) therapy confers a survival benefit compared with IFN-α treatment alone [17,18]. However, the role of nephrectomy in the context of targeted anti-angiogenic therapies has been unclear, although retrospective studies have tended to confirm the benefit of the procedure [19]. At ESMO 2017, data were presented on the sequence nephrectomy → sunitinib versus sunitinib → nephrectomy (SURTIME). Although the study was stopped early because of lengthy patient recruitment, it can be used as a prospective data source. No difference in median PFS at 7 months was shown between treatment groups (42.0% vs. 42.9%, p>0.99), but a trend toward better overall survival (OS) was shown in the group with secondary surgery (HR 0.57, 95% CI 0.34-0.95, p=0.032) [20]. With the recently published phase III CARMENA trial, prospective data are available demonstrating the non-inferiority of sunitinib therapy alone compared with nephrectomy plus sunitinib in intermediate and adverse risk patients [21]. The study randomized 450 patients to treatment with nephrectomy/sunitinib or sunitinib. Patients treated with sunitinib alone showed a longer median OS than the group with nephrectomy (18.4 vs. 13.9 months, HR 0.89) and the criteria of non-inferiority of sunitinib were met accordingly [21]. The authors conclude that although nephrectomy may be useful for symptom control (macrohematuria, renal pain), therapy with a VEGF inhibitor without nephrectomy has no disadvantages in terms of OS [21]. If the risk is low, nephrectomy will probably continue to be chosen for the majority of patients.
Choice of first-line therapy
As first-line therapy, sunitinib and pazopanib have been most commonly used in patients with favorable and intermediate risk, respectively [22]. The data from the CheckMate-214 trial and the approval of nivolumab/ipilimumab will lead to a change in the treatment algorithm. Overall survival data from the IMmotion151 (atezolizumab and bevacizumab vs. sunitinib) trial are also awaited with interest. However, the choice of first-line therapy is also based on each patient’s individual circumstances and any comorbidities [22]. Sunitinib more than doubled median PFS (11 vs. 5 months, HR 0.539, p<0.001) and achieved median OS of more than two years (26.4 vs. 21.8 months, HR 0.821, p=0.051) compared with IFN-α [23]. In the real-world Expanded Access study, sunitinib was also observed to have a median PFS of 9.4 months and a median OS of 18.7 months [24]. After more than ten years of experience, sunitinib has now replaced IFN-α as the standard of care in first-line therapy [25].
In the randomized, phase III, non-inferiority COMPARZ trial, pazopanib was non-inferior to sunitinib (9.5 months) with a median PFS of 8.4 months (HR 1.05). Median OS was also comparable between the two therapies (pazopanib: 28.3 months, sunitinib 29.1 months, HR 0.92), and the objective response rate was 31% under pazopanib vs. 25% under sunitinib [26]. In a randomized patient preference trial (PICES), pazopanib performed statistically significantly better than sunitinib [27]. As another option in first-line therapy, a 3.3-month improvement in median PFS was observed with the combination of bevacizumab with IFN-α compared with IFN-α (8.5 vs. 5.2 months, HR 0.71) [28]. In patients at unfavorable risk, a significant prolongation of median OS was also observed with temsirolimus versus IFN-α (10.9 vs. 7.3 months, HR 0.73) [29].
Choice of second-line therapy and follow-up treatments
In addition to the duration of response to first-line therapy, patient symptomatology, and existing comorbidities, previous individual toxicities also influence the choice of second-line therapy [22,30]. Patients who experienced severe side effects or quality-of-life declines with their previous therapy may therefore benefit from a change of drug class [22]. For example, poorly controlled type 2 diabetes may be worsened by the use of an mTOR inhibitor, cardiovascular disease may be a risk factor in the context of anti-VEGF therapies, and existing autoimmune diseases must be considered in the context of checkpoint inhibitor-based therapies [31–33].
In the phase III AXIS trial, significantly longer median PFS (HR 0.66, p<0.0001) and median OS were achieved with axitinib versus sorafenib in the second line of therapy (20.1 months vs. 19.2 months, HR 0.97, p=0.37) [34]. In addition, compared to everolimus, both cabozantinib in the phase III METEOR trial (21.4 vs. 17.1 months, HR 0.70, p=0.0002) and nivolumab in the phase III CheckMate-025 trial (25.0 vs. 19.6 months, HR 0.73, 98.5% CI 0.57-0.93, p=0.002) resulted in a significant improvement in median OS [35,36]. However, no PFS benefit was shown with nivolumab (4.6 vs. 4.4 months, HR 0.88, p=0.11) [36]. The combination of lenvatinib and everolimus resulted in a significant prolongation of median PFS compared to everolimus (14.6 vs. 5.5 months, HR 0.40, p=0.0005), but associated with toxicities [37,38].
Therapy and side effect management
The success of treatment in mRCC depends on the choice of therapy, but also on the management of side effects.
In the COMPARZ trial, different adverse event profiles were observed with sunitinib and pazopanib, which may influence individual drug selection [26]. Fatigue, hand-foot syndrome, and oral mucositis increased with sunitinib, and altered liver enzyme levels and weight loss increased in the pazopanib arm. Study therapy was discontinued more frequently due to adverse effects in the pazopanib group than in the sunitinib group [26].
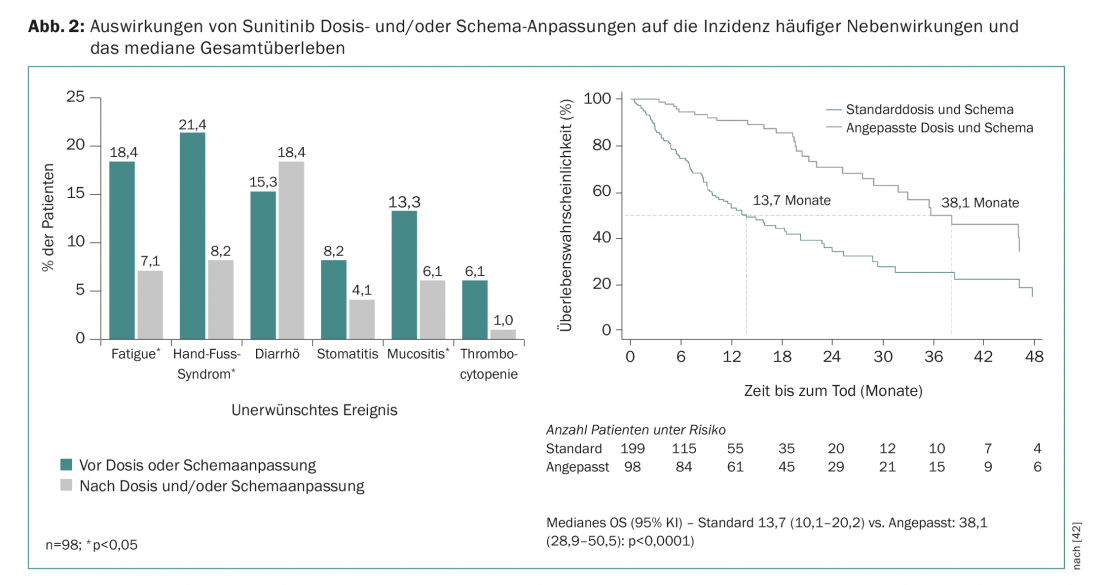
Retrospective pharmacologic analyses indicate that higher sunitinib exposure is associated with better clinical outcomes but also with a higher rate of adverse events [39]. It is now known from various studies that adjusting the sunitinib treatment regimen from “4 weeks of therapy/2 weeks of rest (4/2)” to “2 weeks of therapy/1 week of rest (2/1)” can help reduce the most common adverse effects without compromising the effectiveness of therapy [40,41]. This is also confirmed by data from the prospective, real-world STAR-TOR registry in Germany. Dose and/or regimen adjustments during sunitinib therapy resulted in a reduction in common adverse events (except diarrhea) and an improvement in median OS (Fig. 2) [42]. Dose adjustments are also frequently necessary and common during treatment with pazopanib [43].
Outlook
With the now wide range of treatment options in mRCC and the expected additional agents, the question of optimal therapy choice and sequence remains a key issue. For example, the EuroTARGET cohort is evaluating different biomarkers for personalization of mRCC therapy using data from sunitinib-, pazopanib- or sorafenib-treated patients [44]. In addition, proactive and individualized management of therapy and side effects will continue to have a significant impact on clinical outcomes.
Take-Home Messages
- In intermediate/unfavorable risk, primary palliative nephrectomy has not shown an advantage over primary anti-VEGF therapy in terms of overall survival. If the risk is favorable, primary cytoreductive nephrectomy remains the standard of care.
- In first-line therapy, sunitinib and pazopanib are recommended for favorable/intermediate risk, whereas temsirolimus may be used for unfavorable risk or non-small cell histology.
- Nivolumab/Ipilimumab can be used with cost approval or within the SAKK 07/17 study and is now also approved in Switzerland. Cabozantinib, on the other hand, does not yet have first-line approval.
- Second-line treatment is based on several individual factors as well as response and toxicities with first-line therapy.
- Adjusting the sunitinib treatment regimen from “4 weeks of therapy/2 weeks off (4/2)” to “2 weeks of therapy/1 week off (2/1)” may help improve tolerability without loss of treatment efficacy.
Literature:
- Krebsliga Schweiz: Cancer in Switzerland: important figures. Status Oct. 2017 www.krebsliga.ch/ueber-krebs/zahlen-fakten/-dl-/fileadmin/downloads/sheets/zahlen-krebs-in-der-schweiz.pdf
- Choueiri TK, Motzer RJ: Systemic Therapy for Metastatic Renal-Cell Carcinoma. New England Journal of Medicine 2017; 376(4): 354-366.
- Clark PE: The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney international 2009; 76(9): 939-945.
- Nickerson ML, et al: Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clinical Cancer Research 2008; 14(15): 4726-4734.
- Thomas GV, et al: Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nature medicine 2006; 12(1): 122-127.
- Sathianathen NJ, et al: The current status of immunobased therapies for metastatic renal-cell carcinoma. ImmunoTargets and Therapy 2017; 6: 83-93.
- Rini BI, Small EJ: Biology and Clinical Development of Vascular Endothelial Growth Factor-Targeted Therapy in Renal Cell Carcinoma. Journal of Clinical Oncology 2005; 23(5): 1028-1043.
- Motzer RJ, Bacik J, Murphy BA, Russo P Mazumdar M: Interferon-Alfa as a Comparative Treatment for Clinical Trials of New Therapies Against Advanced Renal Cell Carcinoma. Journal of Clinical Oncology 2002; 20(1): 289-296.
- Heng DYC, et al: External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet. Oncology 2013; 14(2): 141-148.
- Heng DYC, et al: Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor-Targeted Agents: Results From a Large, Multicenter Study. Journal of Clinical Oncology 2009; 27(34): 5794-5799.
- Powles T, et al: Updated European Association of Urology Guidelines Recommendations for the Treatment of First-line Metastatic Clear Cell Renal Cancer. European Urology 2017.
- Motzer RJ, et al: Nivolumab plus ipilimumab versus sunitinib in advanced renal cell carcinoma. New England Journal of Medicine 2018; 378(14): 1277-1290.
- S3 Guideline Diagnosis, Therapy and Follow-up of Renal Cell Carcinoma. Long version 1.2 – April 2017, AWMF register number: 043/017-OL.
- Escudier B, et al: Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology 2016; 27(5): v58-v68.
- NCCN: National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology – Kidney Cancer Version 3.2018. NCCN.org Feb. 6 2018.
- www.swissmedicinfo.ch.
- Flanigan RC, et al: Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. New England Journal of Medicine 2001; 345(23): 1655-1659.
- Mickisch GH, Garin A, et al: Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 2001; 358(9286): 966-970.
- Culp SH: Cytoreductive nephrectomy and its role in the present-day period of targeted therapy. Ther Adv Urol 2015; 7(5): 275-285.
- Bex A, et al: Immediate versus deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma (mRCC) receiving sunitinib (EORTC 30073 SURTIME. Annals of Oncology 2017; 28(5): v605-v649; doi: 10.1093/annonc/mdx440.
- Mejean A, et al: Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. The New England journal of medicine 2018. doi: 10.1056/NEJMoa1803675
- Osorio JC, et al: Optimizing Treatment Approaches in Advanced Renal Cancer. Oncology 2017; 31(12): 919-926, 928-930.
- Motzer RJ, et al: Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. Journal of Clinical Oncology 2009; 27(22): 3584-3590.
- Gore ME, et al: Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. British journal of cancer 2015; 113(1): 12-19.
- Motzer RJ, et al: Sunitinib: Ten Years of Successful Clinical Use and Study in Advanced Renal Cell Carcinoma. The Oncologist 2017; 22(1): 41-52.
- Motzer RJ, et al: Pazopanib versus sunitinib in metastatic renal cell carcinoma. New England Journal of Medicine 2013; 369(8): 722-731.
- Escudier B, et al: Randomized, Controlled, Double-Blind, Cross-Over Trial Assessing Treatment Preference for Pazopanib Versus Sunitinib in Patients With Metastatic Renal Cell Carcinoma: PISCES Study. Journal of Clinical Oncology 2014; 32(14): 1412-1418.
- Rini BI, et al: Bevacizumab Plus Interferon Alfa Compared With Interferon Alfa Monotherapy in Patients With Metastatic Renal Cell Carcinoma: CALGB 90206. Journal of Clinical Oncology 2008; 26(33): 5422-5428.
- Hudes G, et al: Temsirolimus, interferon alfa, or both for advanced renal cell carcinoma. New England Journal of Medicine 2007; 356(22): 2271-2281.
- Fischer S, et al: Sequence of treatment in locally advanced and metastatic renal cell carcinoma. Translational andrology and urology 2015; 4(3): 310-325.
- Johnson DB, et al: Immune Checkpoint Inhibitor Therapy in Patients With Autoimmune Disease. Oncology 2018; 32(4): 190-194.
- Morviducci L, et al: Everolimus is a new anti-cancer molecule: metabolic side effects as lipid disorders and hyperglycemia. Diabetes research and clinical practice 2018.
- Schmidinger M, et al: Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008; 26(32): 5204-5212.
- Rini BI, et al: Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011; 378(9807): 1931-1939.
- Motzer RJ, et al: Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. British journal of cancer 2018; 118(9): 1176-1178.
- Motzer RJ, et al: Nivolumab versus everolimus in advanced renal cell carcinoma. The New England journal of medicine 2015; 373(19): 1803-1813.
- Motzer RJ, et al: Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015; 16(15): 1473-1482.
- Rothermundt C, et al: Second-line treatment for metastatic clear cell renal cell cancer: experts’ consensus algorithms. World journal of urology 2017; 35(4): 641-648.
- Houk BE, et al: Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemotherapy and Pharmacology 2009; 66(2): 357-371.
- Atkinson BJ, et al: Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Treated with Alternative Sunitinib Schedules. The Journal of Urology 2014; 191(3): 611-618.
- Najjar YG, et al: A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. European Journal of Cancer 2014; 50(6): 1084-1089.
- Boegemann M, et al: Treatment modification with sunitinib in first-line (1L) metastatic renal cell carcinoma (mRCC): An analysis of the STAR-TOR registry. Journal of Clinical Oncology 2018; 36(6_suppl): 602-602.
- Iacovelli R, et al: Clinical outcome of patients who reduced sunitinib or pazopanib during first-line treatment for advanced kidney cancer. Urologic oncology 2017; 35(9): 541.e547-541.e513.
- van der Zanden LFM, et al: Description of the EuroTARGET cohort: A European collaborative project on TArgeted therapy in renal cell cancer-GEnetic- and tumor-related biomarkers for response and toxicity. Urologic oncology 2017; 35(8): 529.e529-529.e516.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(4): 17-22.











