The phytotherapeutic agent menthacarin has been used for decades to treat functional gastrointestinal disorders. The effect is based, among other things, on inhibition of visceral hypersensitivity. Current knowledge suggests that visceral hypersensitivity is multifactorial, with TRP ion channels playing a central role. In an animal study, regulation of intracellular calcium signal transduction by menthacarin was shown to be mediated by TRP-ion channels is mediated.
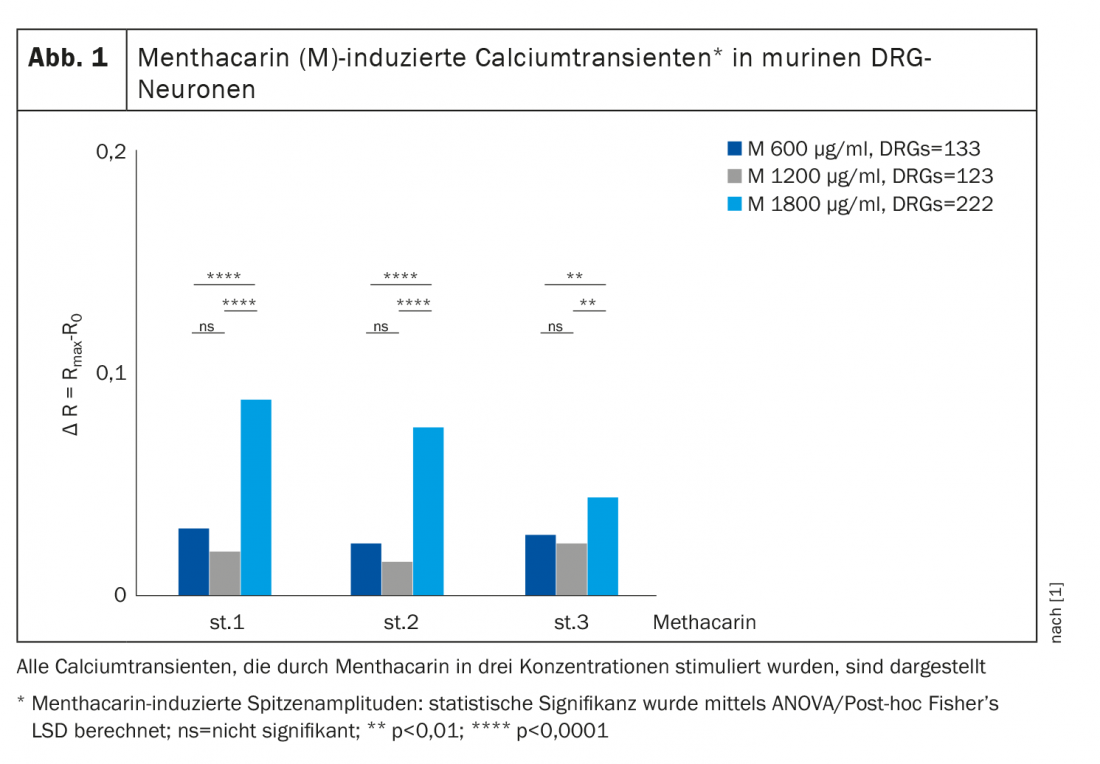
TRP (transient receptor potential) ion channels are highly permeable to divalent calcium ions and targets for the treatment of visceral hypersensitivity [1]. From changes in calcium balance, conclusions can be drawn about the complex interrelationships of visceral nociception as a basis for pharmacotherapeutic interventions. Against this background, Zhang et al. 2021 investigated the effect of menthacarin on calcium influx in spinal ganglion neurons (DRG neurons), peritoneal macrophages, and colon organoids in a mouse model [1]. To this end, they superfused freshly isolated lumbosacral DRG neurons, peritoneal macrophages, and colon organoids cultured over 7 days from wild-type mice (WT/C57BL/6) with menthacarin at various concentrations (600, 1200, and 1800 µg/ml). Calcium signals and the flux of intracellular calcium were measured using the calcium imaging method.
|
Researchers demonstrated that menthacarin at various concentrations induced calcium ion influx in sensory neurons of thedorsal root ganglion(DRG), peritoneal macrophages, and colon organoids [1]. (+)-Carvone, an essential component of caraway oil, acts as a specific agonist of TRPA1 chemosensory channels [2]. TRP ion channels are highly permeable to divalent calcium ions. |
|
Visceral nociception in FGID. Visceral pain is a common feature of functional gastrointestinal disorders (FGIDs). The nociceptors in the gastrointestinal tract have nerve endings in all layers of the gastrointestinal tract (mucosal, submucosal, muscular); their cell bodies are located in the in spinal ganglion neurons (DRG neurons) [9]. The nociceptive signal is connected via the posterior root of the spinal cord and ascending pathways predominantly in the contralateral spinothalamic tract. This signal is transmitted via the thalamocortical system to the cortex of the brain, where the evaluation of pain perception takes place. Visceral hyperalgesia is characteristic of patients with FGIDs. The active ingredient combination menthacarin (Carmenthin®), a high-dose mixture of peppermint and caraway oil has been shown to counteract visceral hypersensitivity [6,10]. |
Desensitization of TRP channels as an important mechanism of action.
Menthacarin was found to induce intracellular calcium transients in DRG (dorsal root ganglion) neurons (Fig. 1), peritoneal macrophages, and colon organoids. In this regard, (+)-carvone, the essential component of caraway oil, acts as a specific agonist of TRPA1 chemosensory channels [2]. In addition, colon organoids and, to a lesser extent, sensory DRG neurons showed decreasing responsiveness (tachyphylaxis) of calcium transients with repeated applications of menthacarin. Previous studies in mouse models show that desensitization/tachyphylaxis of TRP channels is a potential treatment for pain and inflammation [3,4]. This mechanism may also explain the analgesic effects of menthacarin in the clinical setting [5–8]. Of note, organ bath studies in human intestinal tissue demonstrated nerve-independent inhibition of smooth muscle contractility by menthacarin, as evidenced by sustained muscle relaxation and a decrease in phasic contractility. The decrease in contractility was mediated, at least in part, by inhibition of L-type calcium channels [7].
Literature:
- Zhang Z, et al: Life Sci 2021; 264: 118682.
- Kang Q, et al: Biophys Res Commun 2015; 459 (3): 498-503.
- Kistner K, et al: Sci Rep 2016; 6: 28621, https://doi.org/10.1038/srep28621.
- Khalil M, et al: Life Sci 2020; 257, 118112, https://doi.org/10.1016/j.lfs.2020.118112.
- Botschuijver S, et al: Motil 2018; 30 (6) , e13299, https://doi.org/10.1111/nmo.13299
- Adam B, et al: Scandinavian Journal of Gastroenterology 2006; 41: 155-160.
- Krueger D, et al: Motil 2020; 32 (2), e13748, https://doi.org/10.1111/nmo.13748.
- Jacquot L, et al: Rhinology 2005; 43 (2): 93-98.
- Moloney RD, et al: CNS Neurosci Ther 2016; 22(2): 102-117.
- Drug Information, www.swissmedicinfo.ch, (last accessed 07/05/2022).
HAUSARZT PRAXIS 2022; 17(7): 27