Performing flow cytometry from high-quality CSF samples allowed the identification of specific immune cell signatures in different neurological diseases. This has improved the differentiation of various neurological diseases, e.g. immune neuropathies and autoimmune diseases of the central nervous system, and provided insight into possible cell populations involved in the different diseases.
In the Department of Neurology with Institute of Translational Neurology at Münster University Hospital, flow cytometry from high quality CSF samples was performed at an unprecedented scale and a unique, comprehensive database was established that allowed identification of specific immune cell signatures in different neurological diseases. This has improved the differentiation of various neurological diseases, e.g. immune neuropathies and autoimmune diseases of the central nervous system, and provided insight into possible cell populations involved in the different diseases. The following article allows insight into the scientific work based on flow cytometry from cerebrospinal fluid and gives a brief overview of certain immune cell compositions in important neurological diseases.
CSF as a diagnostic window into the central nervous system
The cerebrospinal fluid (CSF for short) is a clear fluid that circulates around the central nervous system (CNS), surrounding the brain and spinal cord. In this context, CSF not only provides mechanical protection, but also likely supplies central nervous system cells with nutrients [1], serves as a transport medium for immune cells [2], and mediates antigen transport between the CNS and peripheral compartments, such as blood [3]. The non-cellular fraction of CSF is produced as an ultrafiltrate from serum in the choroid plexus in the cerebral ventricles, with solutes transferring from serum to CSF in a size-dependent manner. CSF drainage (reabsorption into the circulation) occurs via the venous and lymphatic systems [3,4]. The cells of the CSF are leukocytes, which are called CSF leukocytes or CSF cells. The cell concentration in CSF is about a thousand times lower than in peripheral blood. Under physiological conditions, CSF contains ≤4 CSF leukocytes/µl (or <5/liquor-leukocytes/µl). Thereby, the cellular profile in the CSF is lymphocytic and monocytic and is characterized by a dominance of CD4+ T lymphocytes. Compared with peripheral blood, memory cells and regulatory T lymphocytes and natural killer (NK) cells are increased in CSF, whereas B lymphocytes are reduced and plasma cells are not present under physiological conditions [5,6]. This suggests that the cellular composition of the CSF forms its own compartment, which is subject to strict regulation and control and must be regulated independently of the peripheral blood. Since the CSF surrounds the central nervous system, it forms the compartment closest to the CNS, which is accessible in clinical practice for the purpose of diagnostic analyses by means of lumbar puncture. It is well known that CSF can provide information on the cellular mechanisms of CNS diseases. Immunophenotyping of CSF cells – as we present in this article – may help to gain new insights into pathophysiology, to refine diagnostic analyses, and to better assess individual prognoses. In the future, this may help develop new therapeutic approaches for various diseases of the nervous system [7,8].
Flow cytometry of cerebrospinal fluid cells on an unprecedented scale
Flow cytometry is a method for quantitative detection and molecular characterization of cells. Oriented to cellular properties, this method allows classification into different cellular classes (e.g. lymphocytes, monocytes). In flow cytometry, cells of a suspension are registered in a so-called flow cytometer. Each individual cell passes through a laser beam, from whose “response profile” it is possible to detect the cell size, cell granularity and expression of cell markers of each individual cell. Specific marker combinations determine which cell population is involved (Table 1) [5,14].
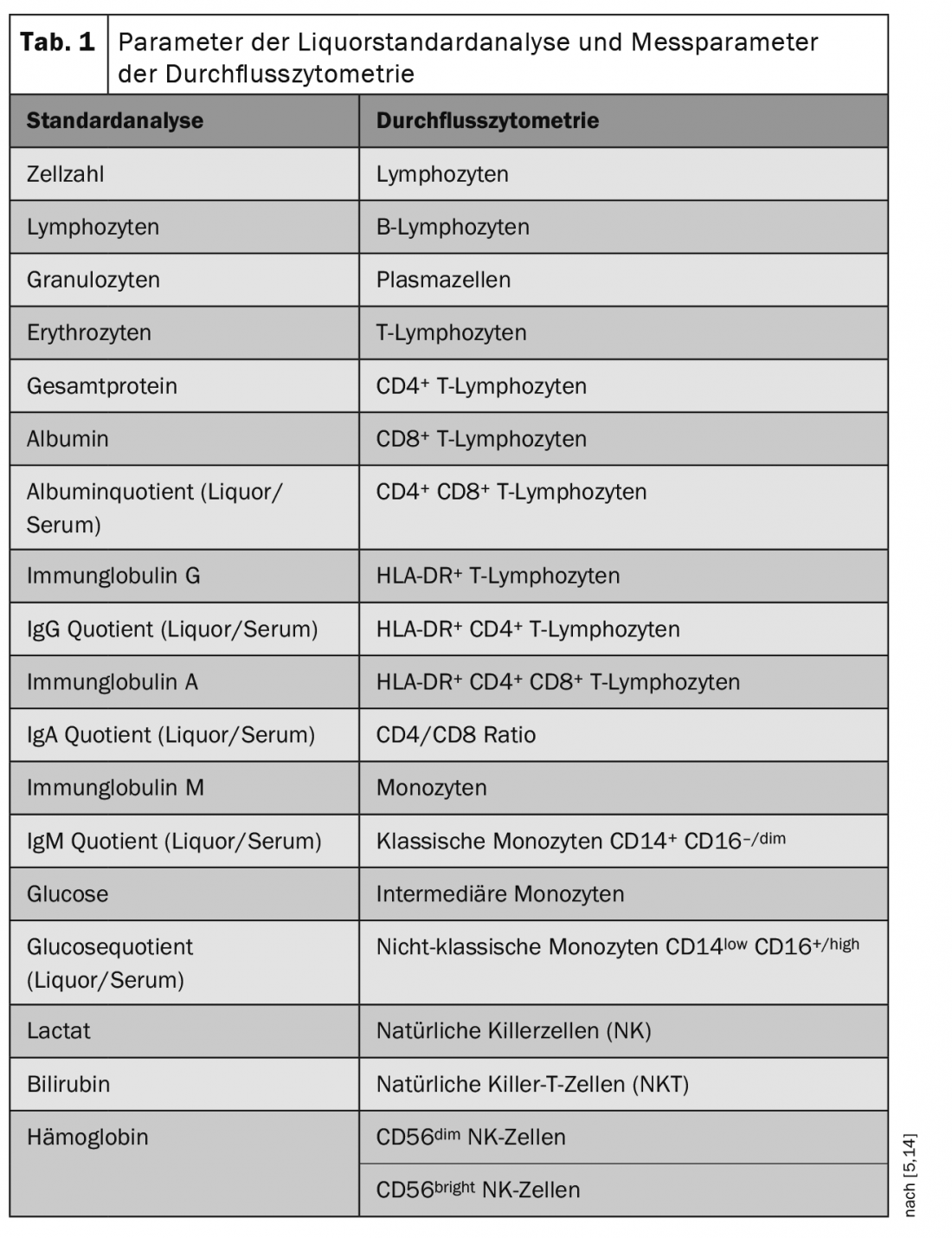
In the CSF laboratory of the Department of Neurology with Institute for Translational Neurology of the University Hospital in Münster, very extensive flow cytometric investigations from blood and in particular also from high-quality CSF samples of patients with various neurological diseases have been performed in recent years. Approximately one thousand CSF samples were analyzed annually by standardized flow cytometry and the data sets were collected in a biobank. These data have thus reached an unprecedented scale and are available to numerous research networks at national as well as international level.
In the first resulting scientific work, immune cells were compared in the cerebrospinal fluid and blood of patients with autoimmune diseases of the central and peripheral nervous systems [5,9]. Furthermore, flow cytometric analysis was used in patients with neuro-degenerative diseases such as Alzheimer’s dementia and frontotemporal dementia [7,10]. In cooperation with the Department of Psychiatry at Münster University Hospital, leukocytes from blood and cerebrospinal fluid samples of patients with primary psychotic disorders were examined and compared with the immune profiles of neurological diseases.
The database of the Department of Neurology with Institute of Translational Neurology at Münster University Hospital will continue to be expanded with new data sets in the future and serve as a source of information for more in-depth analyses and specified differential diagnostics of various diseases.
Neuropathies
Neuropathies – diseases of the peripheral nervous system – are very common and can affect motor, sensory and autonomic nerve fibers. Clinically, neuropathies are differentiated depending on their distribution pattern, clinical course, and genesis. Depending on the localization of affected nerves, a distinction is made between mononeuropathies and polyneuropathies (PNP), among others. A wide spectrum of possible causes of neuropathies exists; including metabolic, toxic, hereditary, infectious, autoimmune, vasculitic, paraproteinemic, and malignancies. The most common cause of polyneuropathy in the Western world is diabetes mellitus, followed by ethyltoxic genesis. Up to 10% of neuropathies are caused by autoimmune processes and are therefore also referred to as immune neuropathies [11].
Diagnosis is primarily based on anamnesis as well as physical and neurological examination. Special attention is paid to infections that have taken place, e.g. gastroenteritis and upper respiratory tract infections, previous illnesses and symptoms of other organ systems such as autonomic symptoms. The first laboratory test is a standard diagnostic test. With the help of the extended diagnosis of certain ganglioside antibodies (GM1, GM2, GQ1b, etc.), the diagnosis of an autoimmune-mediated immune neuropathy can be supported in case of clinical suspicion. Inflammatory parameters, immunoelectrophoresis, rheumatoid antibodies (ANA, AN-CA), or antineuronal antibodies (anti-Hu, anti-CV2/CRMP5, anti-amphiphysin, anti-Ma2) may be indicative of systemic or malignant disease as the cause of inflammatory polyneuropathies. To exclude viral or bacterial infections, a comprehensive history and serological diagnosis of viral (EBV, CMV, HSV, VZV, FSME, HBV, HIV, polio) or bacterial pathogens (Borrelia, Treponema pallidum) can be added. Electrophysiological measurements can detect distribution and damage patterns more accurately and allow classification into different subtypes. Electromyography can be used to identify other myogenic patterns of damage, such as denervation, muscular atrophy, and spontaneous activity. Imaging techniques such as sonography and magnetic resonance imaging can visualize patterns of damage and aid in the diagnosis of exclusion of other causes of neuropathies, e.g., neuronal tumors such as schwannomas.
Immune Neuropathies
Immune neuropathies are particularly relevant because they can be treated if diagnosed early and therapy is started appropriately. Primarily, immune neuropathies are classified into acute (≤four weeks), subacute (four to eight), or chronic (≥eight weeks) course type. Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is known as Guillain-Barré syndrome (GBS) and is the most common form of acute-onset immune neuropathies, whereas chronic inflammatory demyelinating polyneuropathy (CIDP) is the prototype of chronic immune neuropathies. The differential diagnosis of inflammatory polyneuropathies remains a major challenge to this day. In addition to autoimmune-mediated neuropathies, various other causes must be considered for differential diagnosis (Fig. 1).
Differentiation of immune neuropathies using immune cell profiles.
The classification into acute and chronic immune neuropathies is partly artificial and there are many overlaps and smooth transitions between the two forms. Thus, CIDP shows an acute GBS-like onset in up to 10% of cases. Conversely, a subacute course lasting more than four weeks has been described in courses initially classified as GBS [12]. To date, no prognostic parameters exist to predict an acute vs. chronic course at the time of initial clinical manifestation. Because treatment options and response to different immunomodulatory therapies differ in GBS and CIDP, early differentiation of the entities is important and could have a positive impact on disease progression and prognosis.
In standard CSF measurements in inflammatory neuropathies, a so-called “cytalbuminous dissemination” with increased total protein in the CSF can be detected with a normal cell count and often a blood-brain barrier disorder.
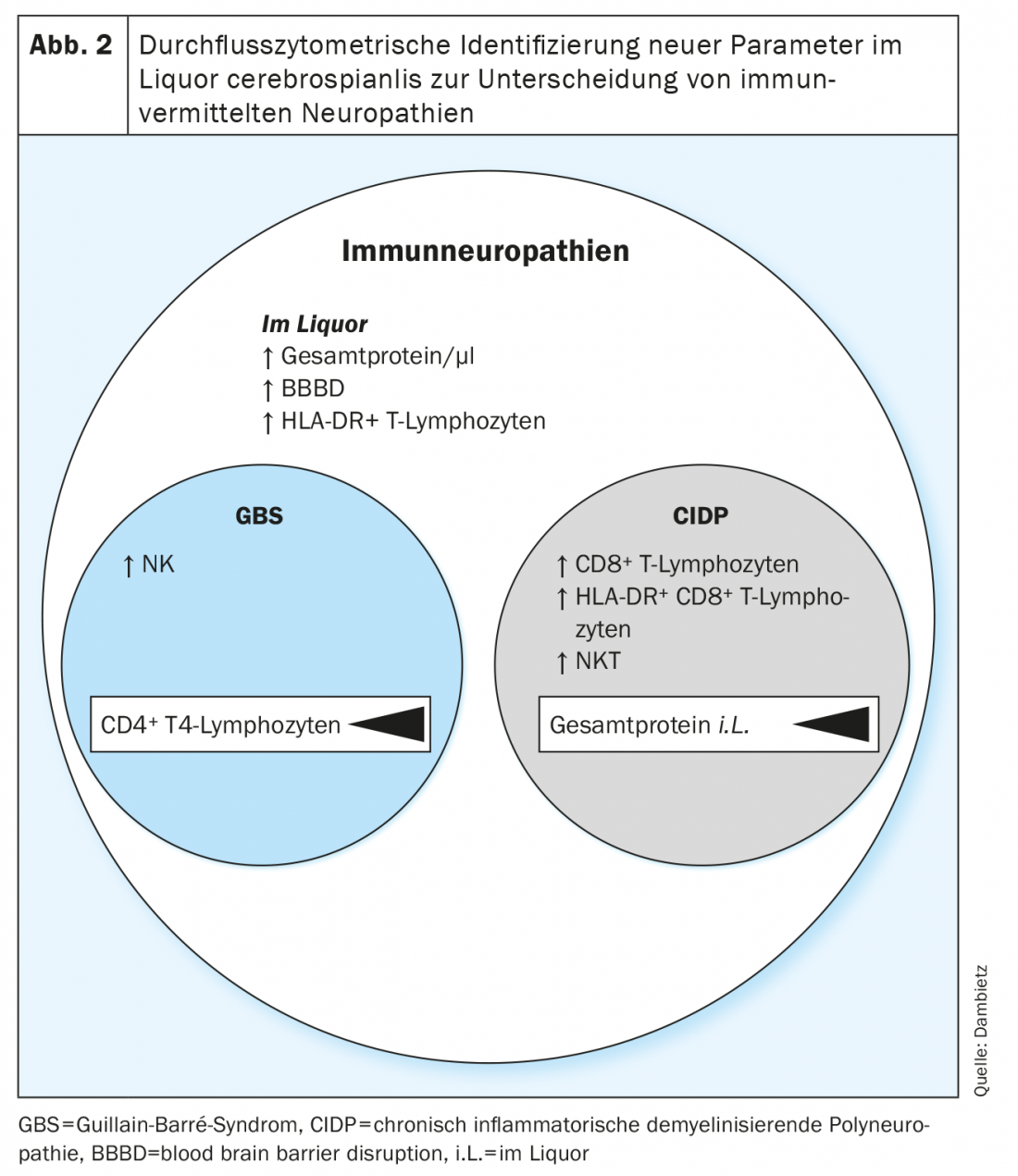
Now, for the first time, by using flow cytometry, the immune cell compositions in the CSF of patients with GBS and CIDP were retrospectively analyzed and compared [13]. The heterogeneous profiles provided evidence for the involvement of different cell populations in the pathophysiology of acute vs. chronic immune neuropathies.
Flow cytometry registered increased numbers of activated and nonactivated (HLA-DR+ vs. HLA-DR-) T lymphocytes in the CSF of patients with inflammatory neuropathies. However, T-cell activation as well as increased CSF protein and blood-brain barrier disruption are nonspecific parameters for neuropathies and are poorly suited to distinguish GBS vs. CIDP, as T-lymphocyte activation is frequently seen in acute as well as chronic courses of neuropathies and is also detectable in other neurologic diseases [14,15].
More specifically, an increased proportion of natural killer (NK) cells was detected in the CSF of Guillain-Barré syndrome, whereas in chronic inflammatory demyelinating polyneuropathy, the number of natural killer T cells (NKT) and CD8+ T lymphocytes was increased (Fig. 2). In a direct comparison of Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy, the detection of natural killer T cells followed by classical and intermediate monocytes was defined as the highest confidence parameter for differentiating the two neuropathic entities. Immunoprofiling with the different proportions of NK, NKT, and CD8+ T cells reveals disease- and subtype-specific patterns of immune cells in CSF and provides a clue to the involvement of distinct cytotoxic cell types in the pathophysiology of acute vs. chronic-onset immune neuropathies. In addition, the individual cell profiles could be used to prospectively assess the acute versus chronic course at the time of the initial clinical manifestation, thus facilitating the choice of therapy.
The treatment of choice for Guillain-Barré syndrome is plasmapheresis. In addition, intravenous immunoglobulins are equally available as a therapeutic option. Due to a ubiquitous immunoglobulin deficiency, these are nowadays mostly used only secondarily in clinical practice and are applied in particular for therapy escalation after plasmapheresis with insufficient improvement of the clinical symptoms. While corticosteroids showed no effect in GBS [16], good efficacy was demonstrated in chronic inflammatory demyelinating polyneuropathy, which is why they are used as standard in the treatment of CIDP [17–19].
Therapy-naïve GBS and CIDP patients, compared with previously treated patients, showed no significant difference in their liquor-chemical immune cell profiles. Ultimately, this means that flow cytometric differentiation of inflammatory neuropathies can also occur during therapy and the subtype-specific immune cell profiles in the CSF are not distorted by the use of immunoglobulins or corticosteroids, for example.
To highlight the clinical relevance of the subtype-specific immune cell compositions, parameters reflecting the clinical manifestation of the neruopathies were detected. Disease severity was based on the Hughes Disability Score and the modified Rankin Scale (mRS). In Guillain-Barré syndrome, a correlation was shown between the number of CD4+ T lymphocytes as well as non-classical monocytes and the clinical severity of the disease. In CIDP, the level of total protein in CSF correlated with disease severity. Flow cytometry of CSF has established a new diagnostic approach to identify disease- and subtype-specific changes in autoimmune-mediated inflammatory neuropathies, facilitating the differentiation of CIDP from GBS. In the future, this could be the basis for prognostic assessment and development of new therapeutics, and extend immune cellular analysis to other neuropathic variants.
Immunophenotypes in the spectrum of neurological diseases.
Based on the increase in information provided by the use of flow cytometry in different compartments (blood and CSF), data collection was extended to a broader spectrum of neurological diseases. A retrospective cross-sectional study was performed to investigate immune cell compositions in patients with autoimmune-mediated, neurodegenerative, and vascular diseases. As with the immune neuropathies, autoimmune diseases of the central nervous system represent a heterogeneous group of neuroinflammatory pathologies that still require more specific assignment and more precise differential diagnosis and whose underlying pathomechanisms have not yet been fully deciphered.
Flow cytometry was used to detect individual parameters, and multidimensional analyses were used to extract those features that best differentiate neuroinflammatory diseases from neurodegenerative and vascular pathologies [5].
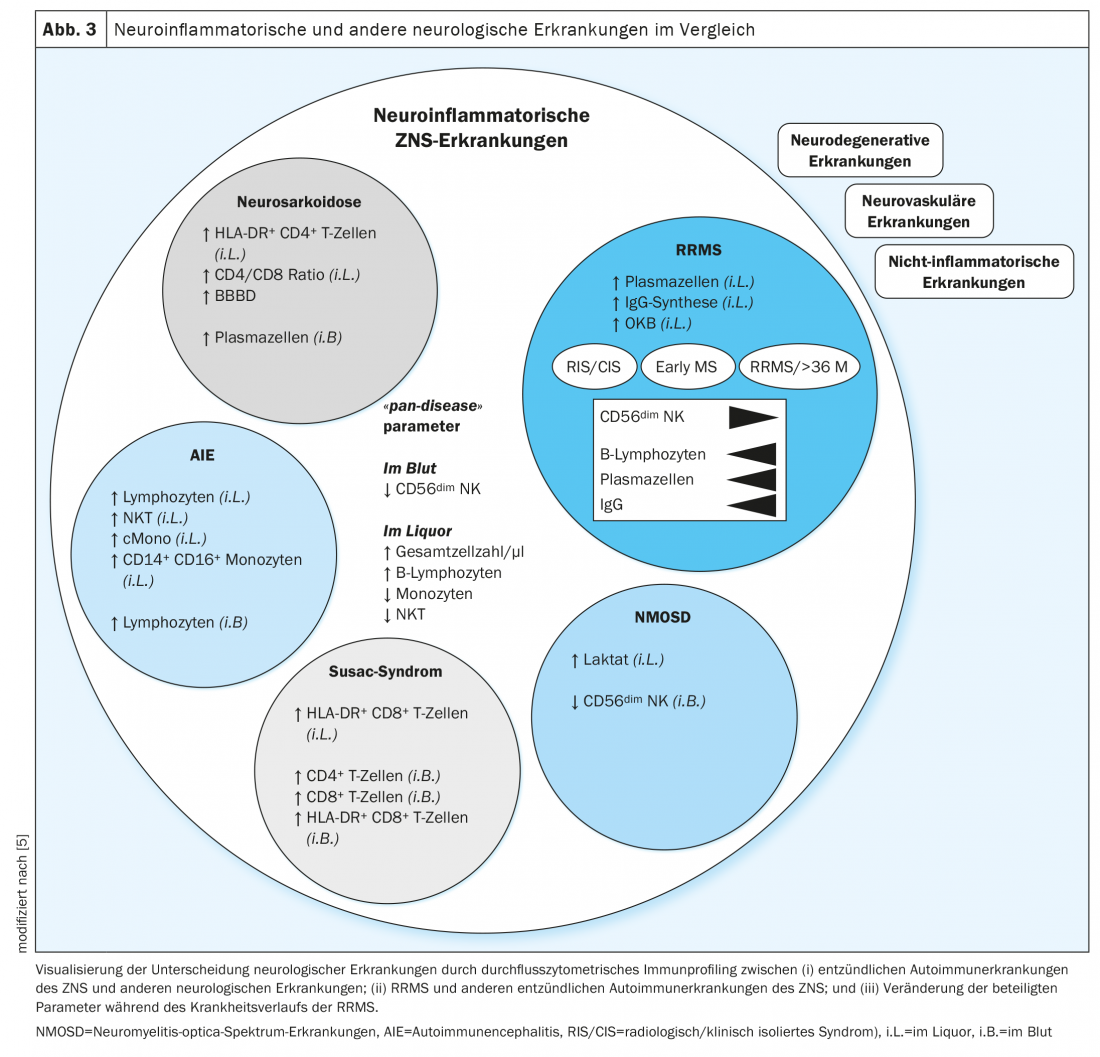
The immune cell profiles of autoimmune neuroinflammatory diseases showed similarities among themselves and differences compared with neurodegenerative, vascular, and non-inflammatory diseases (Fig. 3). In the immune cell profiles of all neuroinflammatory diseases, the cell number as well as the percentage of B lymphocytes in the CSF were increased, whereas monocytes, natural killer cells, and natural killer T cells were decreased. In addition, a decreased number of CD56dim NK cells in the blood was found to be suggestive of the presence of inflammatory CNS disease. These features were defined as “pan-disease” parameters for autoimmune pathologies of the central nervous system, thus indicating changes across diseases (Fig. 3) . In multiple sclerosis, an increase in the number of cells in the CSF and a marked expansion of cells of the B-cell lineage have been known for many years [20,21]. Interestingly, these parameters were amenable to immunotherapies and could be used in the future as biomarkers of response to immunomodulatory therapies and improve prognostic assessment and therapy management.
Immune cell compositions in autoimmune neuroinflammatory diseases.
Disease-specific immune cell signatures in CSF have also been studied in several other neurologic diseases.
A retroperspective study compared immune cell compositions in relapsing-remitting multiple sclerosis (RRMS) and neurosarcoidosis. Sarcoidosis is an autoimmune multisystem disease that manifests with epithelioid granulomas in diverse organs, where macrophage infiltration causes local inflammatory responses [22]. In about 25% of patients, the central nervous system is also affected [23]. This often manifests with neurological symptoms that may be similar to those of an MS relapse. The diagnosis of sarcoidosis includes a blood test with determination of inflammatory parameters, immunoglobulin G, ACE, soluble IL-2 receptor (sIL-2R), and neopterin, as well as pulmonary function testing, bronchoscopy with bronchoalveolar lavage and transbronchial biopsy, chest x-ray, and, depending on the clinical manifestation, other imaging and interdisciplinary studies [24,25]. With regard to differentiation from other inflammatory diseases, laboratory chemical parameters (ACE, sIL-2R) have been shown to be nonspecific and insufficiently sensitive [26,27]. The distinction between neurosarcoidosis and multiple sclerosis is also not always clear on imaging, as MS lesions (white matter lesions) and the granulomatous changes in neurosarcoidosis often resemble each other on magnetic resonance imaging [28]. “Simple” CSF standard analysis also shows similar abnormalities, such as mild pleocytosis [29]. The final diagnosis of neurosarcoidosis can then be made in part only by biopsy from granuloma-suspected lesions in the central nervous system [30]. However, such a biopsy is an invasive and risky procedure and should only be performed after a careful risk-benefit assessment. To avoid such an invasive measure, the establishment of new diagnostic tools is essential. Flow cytometry from CSF and blood of patients with neurosarcoidosis and multiple sclerosis was retrospectively evaluated under this aspect.
In flow cytometry, neurosarcoidosis was surprisingly well distinguished from multiple sclerosis by an increased proportion of plasma cells, intermediate as well as non-classical monocytes in the blood, whereas the number of T-lymphocytes in the blood was decreased in neurosarcoidosis. The CSF showed partly overlapping parameters in both diseases, whereas specifically in neurosarcoidosis the proportion of CD4-positive T lymphocytes was increased. Multidimensional integration of the immunological cell compositions from CSF and blood optimized the differentiation of the two diseases (Fig. 3). The dominance of activated CD4-positive T lymphocytes and an increased CD4/CD8 ratio in the CSF as well as an increased proportion of plasma cells in the blood were defined as characteristics of neurosarcoidosis [14]. Leading the way to multiple sclerosis was the detection of plasma cells in the CSF as well as intrathecal IgG synthesis and oligoclonal bands.
The individual immune cellular compositions in the CSF thus allow a better differentiation of these two inflammatory diseases of the central nervous system and may allow the omission of bioptic diagnostic confirmations in clinical practice and simplify the choice of therapy (classical immunosuppressants vs. MS-modulatory therapies).
Furthermore, we focused on distinguishing distinct autoimmune diseases of the central nervous system and compared immune cell profiles in multiple sclerosis, neuromyelitis optica spectrum disorders (NMOSD), autoimmune encephalitis (AIE), and Susac syndrome. NMOSD is characterized by recurrent optic neuritis and myelitis with long-lasting inflammatory lesions [31]. Autoimmune encephalitides can manifest clinically with headache, fever, vigilance reduction, epileptic seizures, but also with cognitive deficits. NMDA receptor encephalitis (NMDARE) often presents with cognitive deficits, mnestic disorders, and psychotic symptoms [32, 33]. Susac syndrome is a CD8+ T-cell-mediated endotheliopathy of the CNS small blood vessels and is clinically characterized by a triad of encephalopathy, hearing loss, and visual loss [34,35].
While these diseases showed similar changes among themselves in standard analyses of CSF [36], flow cytometry revealed subtype-specific changes in immune cell composition.
In addition to the previously known intrathecal IgG synthesis, intrathecal IgA and IgM synthesis have also been identified as characteristic features of RRMS. In neuromyelitis optica spectrum disorders, in particular, a decreased number of CD56bright NK cells in the blood and an increased lactate concentration in the cerebrospinal fluid were shown. Susac syndrome was characterized by the detection of CD4+ and CD8+ T lymphocytes in the blood and an increased proportion of activated (HLA-DR+) CD8+ T lymphocytes in the CSF as well as in the blood. Characteristic of autoimmune encephalitis was the detection of increased lymphocyte counts in the CSF and blood, as well as increased numbers of Natural T-killer cells, classical monocytes, and CD14+ CD16+ monocytes in the CSF.
Thus, with the help of flow cytometric immune cell analysis from CSF, RRMS can be differentiated from the other entities with high confidence. In a multidimensional analysis, plasma cells in CSF and intrathecal IgG synthesis were defined as the best parameters to differentiate RRMS from other autoimmune CNS diseases.
In depth, the immune cell profiles in patients with multiple sclerosis of relapsing-remitting course type at different stages of the disease were considered. Regardless of disease stage, ‘pan-disease’ parameters were altered to the same degree. In contrast, with progression of the disease, there was a further reduction in CD56dim NK cells in the blood and an increase in intrathecal B lymphocytes, plasma cells, and intrathecal IgG synthesis. (Fig. 3). With the help of immune cell-specific parameters that correlate with disease severity and progression, continuous monitoring could define new markers that facilitate the assessment of disease activity and enable early therapy modulation to positively influence disease progression. Additional data from longitudinal studies over several decades could identify parameters that facilitate the detection of transition from relapsing-remitting to secondary progressive MS. Thus, prospective confirmatory studies are still needed.
The precise analysis of variable immune cell profiles in different disease entities should help new insights into common and distinct pathomechanisms in individual diseases in the future, but the identification of further discriminating parameters requires larger cohorts, especially of patients with rare diseases such as NMOSD or Susac syndrome. In the future, flow cytometry will not be limited to the field of neurology, but will be extended to other medical disciplines.
Immune cell analyses in the interdisciplinary field
Numerous neuroinflammatory diseases can also manifest with psychiatric symptoms, including autoimmune encephalitides such as NMDA receptor encephalitis (NMDARE). Misdiagnoses that classify AIE as a primary disorder from the group of psychiatric disorders occur repeatedly. The resulting consequences can be severe. Currently, the differential diagnosis between psychiatric and neuroinflammatory autoimmune diseases is still a challenge in the clinical routine of neurology and psychiatry. Furthermore, the spectrum of psychiatric disorders also includes numerous entities whose pathogenesis is not yet fully understood [37]. In recent years, immunological and autoimmune mechanisms have also been discussed, which may also contribute to the development of psychiatric disorders [38–41]. For this reason, we analyzed flow cytometric data in the context of psychiatric disorders. In the diagnosis of psychiatric diseases, cerebrospinal fluid analysis has so far been used mainly to exclude organic diseases.
In a retroperspective study, data were collected on immune cell composition in disorders from the primary psychosis form group (including schizophrenia, delusional disorders, acute and transient psychotic disorders, and schizoaffective disorders) and compared with immune cell signatures of neuroinflammatory disorders, particularly NMDARE – a common subtype of AIE.
With the help of flow cytometry, parameters were detected and multidimensional scores were created, which not only serve the exclusion diagnosis, but also support and facilitate the diagnosis of primary psychotic disorders. In addition, the composition of specific immune cells provides evidence for the involvement of certain subpopulations in the genesis and pathological mechanisms of psychiatric disorders. Previous research has shown evidence of increased leukocyte counts in the blood of patients with psychotic disorders [42]. Standard CSF analyses have shown elevation of CSF protein and often the presence of blood-brain barrier dysfunction in primary psychoses. However, specific laboratory and liquor chemistry parameters do not exist, and reliable differentiation from neurological diseases by standard blood and CSF tests has not been possible.
Flow cytometry revealed compartment-specific changes in CSF and blood in patients with primary psychoses [43]. The specific composition of immune cells in CSF in primary psychotic disorders was characterized by increased CD8-positive T lymphocytes and monocytes, especially nonclassical monocytes. In contrast, the number of lymphocytes in the CSF was decreased (overview 1) . The blood showed an increased number of natural killer cells and monocytes. Specifically, the number of classical monocytes was increased, while the percentage of intermediate monocytes in the blood was decreased. NMDA receptor encephalitis (NMDARE) is often associated with psychotic symptoms and can be difficult to distinguish clinically from primary psychosis. Despite the ability to determine specific anti-NMDA receptor antibodies, misdiagnosis is possible because variants of antibody-negative autoimmune encephalitides also exist [44]. For this reason, the two diseases were compared in particular. Flow cytometric immune cell profiling has shown that NMADRE can be readily distinguished from primary psychotic disorders by an increased proportion of lymphocytes, B cells, and monocytes in the CSF and the detection of plasma cells in the blood (Overview 1). Furthermore, flow cytometry detected increased levels of Natural Killer Cells in the blood of patients with positive psychotic symptoms.
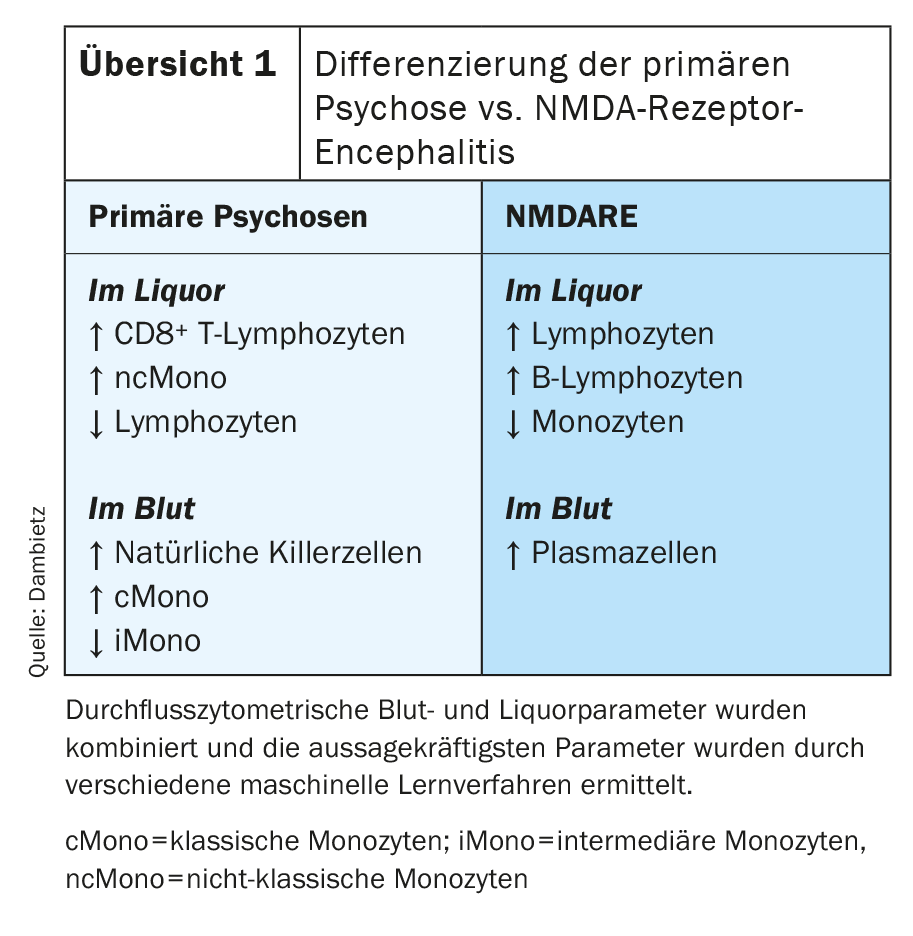
Primary psychotic disorders represent a heterogeneous group whose complex pathophysiology is still misunderstood and unexplored in detail in many aspects. Prior research indicates immune system dysregulation and inflammatory components in the central nervous system in patients with primary psychotic disorders. The flow cytrometric results show changes in the composition of immune cells in the CSF and suggest that immunological mechanisms, especially innate immunity, together with impaired barrier function of the blood-brain barrier contribute to the development of primary psychotic disorders. Flow cytometry has identified disease-typical changes in the composition of immune cells in the CSF and blood that underpin the diagnosis of primary psychosis and support the immune hypothesis of primary psychosis.
Summary
With the help of flow cytometry from CSF, the possibility of novel analyses of disease- and subtype-specific immune cells was established. Flow cytometric CSF studies have allowed specific classification of neuroinflammatory diseases of the central and peripheral nervous systems. Immune cell compositions in the CSF of GBS vs. CIDP patients can distinguish the two entities with higher confidence. In addition, early assessment of disease progression (acute-monophasic vs. chronic-recurrent) can be made at the time of initial diagnosis of inflammatory neuropathy.
In addition, autoimmune inflammatory diseases of the central nervous system exhibit individual changes in cellular subpopulations in the blood as well as in the cerebrospinal fluid. In the future, these disease-specific variations should improve classification into the individual entities and thus facilitate differential diagnosis. Flow cytometric analysis from cerebrospinal fluid can be particularly useful in the case of unclear findings in other examinations, such as imaging, or in the case of nonspecific laboratory chemical changes. For example, when detecting nonspecific lesions on magnetic resonance imaging, e.g., ischemic lesions vs. “white matter lesions” (in MS, NMOSD, or Susac syndrome), or central nervous system granulomas vs. MS lesions, immune cell analysis can be added to the diagnosis.
Furthermore, an early diagnosis can pave the way for therapeutic decisions, such as the use of immunomodulatory drugs, and the influence on the course of the disease can be optimized by a decided choice of therapy. The goal of future flow cytometric CSF analyses could be the detection of immune cellular variables that reflect therapeutic effects and allow a better assessment of disease progression.
Furthermore, the recognition of disease-specific immune cell signatures points the way to the involvement of specific cell populations in the development of neurological diseases and in autoimmune mechanisms in the central and peripheral nervous system. It thus forms the basis for more in-depth analyses of pathophysiological processes.
In summary, flow cytometry from CSF represents a new diagnostic option for the analysis of specific immune cell profiles. Flow cytometry of patients with immune neuropathies, autoimmune neuroinflammatory CNS disorders, or psychotic disorders has revealed disease-specific immune cellular changes in peripheral and intrathecal compartments, suggesting the involvement of the innate immune system and immunological mechanisms in the etiology of the diseases. Specifically, it has been shown that not only cellular components in the blood but also specific immune cells in the CSF play a crucial role in the pathophysiology of neurological diseases.
With the establishment of a unique database of flow cytometric data from high quality CSF samples, the basis for future, more in-depth research projects has been created in the Department of Neurology at Münster University Hospital.
By identifying singular parameters and establishing multifactorial scores, disease- and subclass-specific immune cell profiles could be generated in the future, which could be used as novel diagnostic tools in clinical practice and revolutionize diagnostics as well as therapy of immune neuropathies and other diseases.
Take-Home Messages
- Flow cytometry of CSF cells allows identification of disease-specific immune cell profiles.
- Certain leukocyte populations have been identified as markers of individual diseases and may be involved in individual pathomechanisms, for example, natural killer T cells to differentiate acute vs. chronic immune neuropathies. Monocytes in CSF could distinguish primary psychosis from NDMA receptor encephalitis.
- Prospective CSF cell analyses could improve the selection and monitoring of therapies in clinical practice.
- Flow cytometry of CSF cells can improve the diagnosis of diseases with nonspecific clinical manifestations and overlapping symptoms, thereby facilitating treatment decisions.
Literature:
- Iliff JJ, et al: A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4(147): 147ra111.
- Schlager C, et al: Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 2016; 530(7590): 349-353.
- Engelhardt B, Vajkoczy P, Weller RO: The movers and shapers in immune privilege of the CNS. Nat Immunol 2017; 18(2): 123-131.
- Louveau A, et al: Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523(7560): 337-341.
- Gross CC, et al: Classification of neurological diseases using multi-dimensional CSF analysis. Brain, 2021. 144(9): 2625-2634.
- Han S, et al: Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol 2014; 192(6): 2551-2563.
- Pawlowski M, et al: Relevance of raised cerebrospinal fluid monocyte levels in patients with frontotemporal dementia. Neurobiol Aging 2018; 62: 45-52.
- Alvermann S, et al: Immunophenotyping of cerebrospinal fluid cells in multiple sclerosis: in search of biomarkers. JAMA Neurol 2014; 71(7): 905-912.
- Gross CC, et al: Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci USA 2016; 113(21): E2973-2982.
- Lueg G, et al: Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol Aging 2015; 36(1): 81-89.
- Wiendl HK, Kieseier B, Meuth S: Questions and answers in neuroimmunology 2015: 191-215.
- van Doorn PA: Diagnosis, treatment and prognosis of Guillain-Barre syndrome (GBS). Press Med 2013; 42(6 Pt 2): e193-201.
- Heming M, et al: Immune Cell Profiling of the Cerebrospinal Fluid Provides Pathogenetic Insights Into Inflammatory Neuropathies. Front Immunol 2019; 10: 515.
- Heming M, et al: Leukocyte profiles in blood and CSF distinguish neurosarcoidosis from multiple sclerosis. J Neuroimmunol 2020; 341: 577171.
- Li S, et al: IL-17 and IL-22 in cerebrospinal fluid and plasma are elevated in Guillain-Barre syndrome. Mediators Inflamm 2012; 2012: 260473.
- Double-blind trial of intravenous methylprednisolone in Guillain-Barre syndrome. Guillain-Barre Syndrome Steroid Trial GrouLancet, 1993. 341(8845): 586-590.
- Kieseier BC, et al: Immune-mediated neuropathies. Nat Rev Dis Primers 2018; 4(1): 31.
- Nobile-Orazio E, Gallia F: Update on the treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Curr Opin Neurol 2015; 28(5): 480-485.
- Nobile-Orazio E, et al: Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol 2012; 11(6): 493-502.
- Kraus J, et al: CD45RA+ ICAM-3+ lymphocytes in cerebrospinal fluid and blood as markers of disease activity in patients with multiple sclerosis. Acta Neurol Scand 2000; 102(5): 326-332.
- Cepok S, et al: Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain 2001; 124(Pt 11): 2169-2176.
- Chen ES, Moller DR: Sarcoidosis – scientific progress and clinical challenges. Nat Rev Rheumatol 2011; 7(8): 457-467.
- Iannuzzi MC, Rybicki BA, Teirstein AS: Sarcoidosis. N Engl J Med 2007; 357(21): 2153-2165.
- Bradshaw MJ, et al: Neurosarcoidosis: Pathophysiology, Diagnosis, and Treatment. Neurol Neuroimmunol Neuroinflamm, 2021. 8(6).
- Seve P, et al: Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis, Cells 2021; 10(4).
- Bharwani KD, et al: Elevated Plasma Levels of sIL-2R in Complex Regional Pain Syndrome: A Pathogenic Role for T-Lymphocytes? Mediators Inflamm 2017: 2764261.
- Cai B, et al: Micro-inflammation characterized by disturbed Treg/Teff balance with increasing sIL-2R in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2013; 121(4): 214-219.
- Spencer TS, et al: Clinical and magnetic resonance imaging manifestations of neurosarcoidosis. Semin Arthritis Rheum 2005; 34(4): 649-661.
- Pawate S, Moses H, Sriram S: Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM 2009; 102(7): 449-460.
- Wegener S, et al: Clinically isolated neurosarcoidosis: a recommended diagnostic path. Eur Neurol 2015; 73(1-2): 71-77.
- Jarius S, Wildemann B, Paul F: Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol 2014; 176(2): 149-164.
- Dalmau J, Graus F: Antibody-Mediated Encephalitis. N Engl J Med 2018; 378(9): 840-851.
- Dalmau J, et al: An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol 2019; 18(11): 1045-1057.
- Vishnevskia-Dai V, et al: Susac syndrome: clinical characteristics, clinical classification, and long-term prognosis. Medicine (Baltimore), 2016; 95(43): e5223.
- Gross CC, et al: CD8(+) T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat Commun 2019; 10(1): 5779.
- Toledano M, Weinshenker BG, Solomon AJ: A Clinical Approach to the Differential Diagnosis of Multiple Sclerosis. Curr Neurol Neurosci Rep 2015; 15(8): 57.
- Fritz B: On the serological diagnosis of schizophrenia from cerebrospinal fluid by the method of Lehmann-Facius. 1939/12. 165: 462-467.
- van Kesteren CF, et al: Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 2017; 7(3): e1075.
- Mazza MG, et al: Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World J Biol Psychiatry 2020; 21(5): 326-338.
- Fernandez-Egea E, et al: Peripheral Immune Cell Populations Associated with Cognitive Deficits and Negative Symptoms of Treatment-Resistant Schizophrenia. PLoS One 2016; 11(5): e0155631.
- Doorduin J, et al: Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50(11): 1801-1807.
- Steiner J, et al: Innate Immune Cells and C-Reactive Protein in Acute First-Episode Psychosis and Schizophrenia: Relationship to Psychopathology and Treatment. Schizophr Bull 2020; 46(2): 363-373.
- Rauber S, et al: Cerebrospinal fluid flow cytometry distinguishes psychosis spectrum disorders from differential diagnoses. Mol Psychiatry 2021; 26(12): 7661-7670.
- Dalmau J, et al: Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7(12): 1091-1098.
InFo NEUROLOGY & PSYCHIATRY 2022; 20(3): 6-14.