Avatrombopag is an effective and safe second-line treatment for adult patients with chronic immune thrombocytopenia (ITP). In addition, this thrombopoietin receptor agonist can be used in adults with chronic liver disease prior to invasive surgery to prevent bleeding events. For both indications, treatment should be initiated and supervised by a physician experienced with hematologic disorders.
A reduced platelet count with values <150’000/µl is called thrombocytopenia. This is associated with an increased risk of excessive bleeding, especially after surgery [1]. The etiologic spectrum of thrombocytopenia is broad. Immune thrombocytopenia is an acquired autoimmune disease due to immune-mediated platelet destruction and impaired platelet production. It is estimated that two to five people per 100,000 in the general population are affected by this disease, also known as Werlhof’s disease [2]. Thrombocytopenia is also prevalent in patients with chronic liver disease (CLD) with a clustered occurrence in severe CLD [3]. Other possible causes of thrombocytopenia range from pregnancy to certain cancers and cancer treatments to aplastic anemia and certain viral infections [1]. In Switzerland, avatrombopagum (Doptelet®) has been approved since November 2021 for the treatment of adult patients with chronic immune thrombocytopenia who have responded inadequately to at least one prior treatment and for severe thrombocytopenia in the setting of chronic liver disease when invasive surgery is planned [4,6].
Doptelet® shows significant improvement in platelet response
The drug, available in a peroral dosage form, proved effective in both indications and was generally well tolerated. In phase III trials, avatrombopag was associated with significantly greater platelet response than placebo in patients with chronic immune thrombocytopenia (ITP) and was also shown to be superior in reducing the need for platelet transfusions or “rescue” procedures for bleeding resulting from surgery, in patients with a platelet count <50 ×109/l at baseline [3]. Longer-term data suggest that avatrombopag is associated with high durable response rates in ITP and may have corticosteroid-sparing effects. Avatrombopag binds in the transmembrane domain of the thrombopoietin receptor [5]. In ITP patients, dosing and monitoring recommendations are a bit more complicated than in patients with chronic liver disease (CLD), but again, there are things to keep in mind.
What to consider when treating CLD patients.
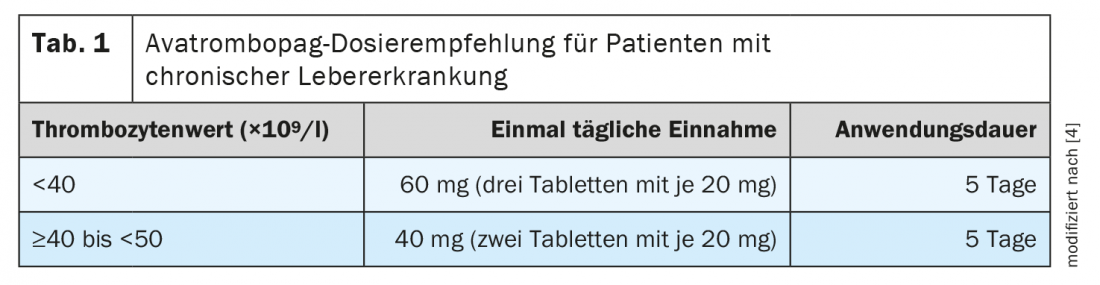
In patients with chronic liver disease, platelet levels must be determined before taking avatrombopag and on the day of a procedure to ensure that there is no unexpectedly large increase in platelet levels. The recommended daily dose of avatrombopag is based on the patient’s platelet counts (Table 1) [4]. Avatrombopag should be started 10 to 13 days before the scheduled surgery. The invasive procedure should be scheduled in the time window of 5 to 8 days after the last dose of avatrombopag. Based on limited information, it is recommended that avatrombopag not be taken for more than 5 days [4].
Werlhof’s disease: specific dosing regimens and monitoring recommendations.
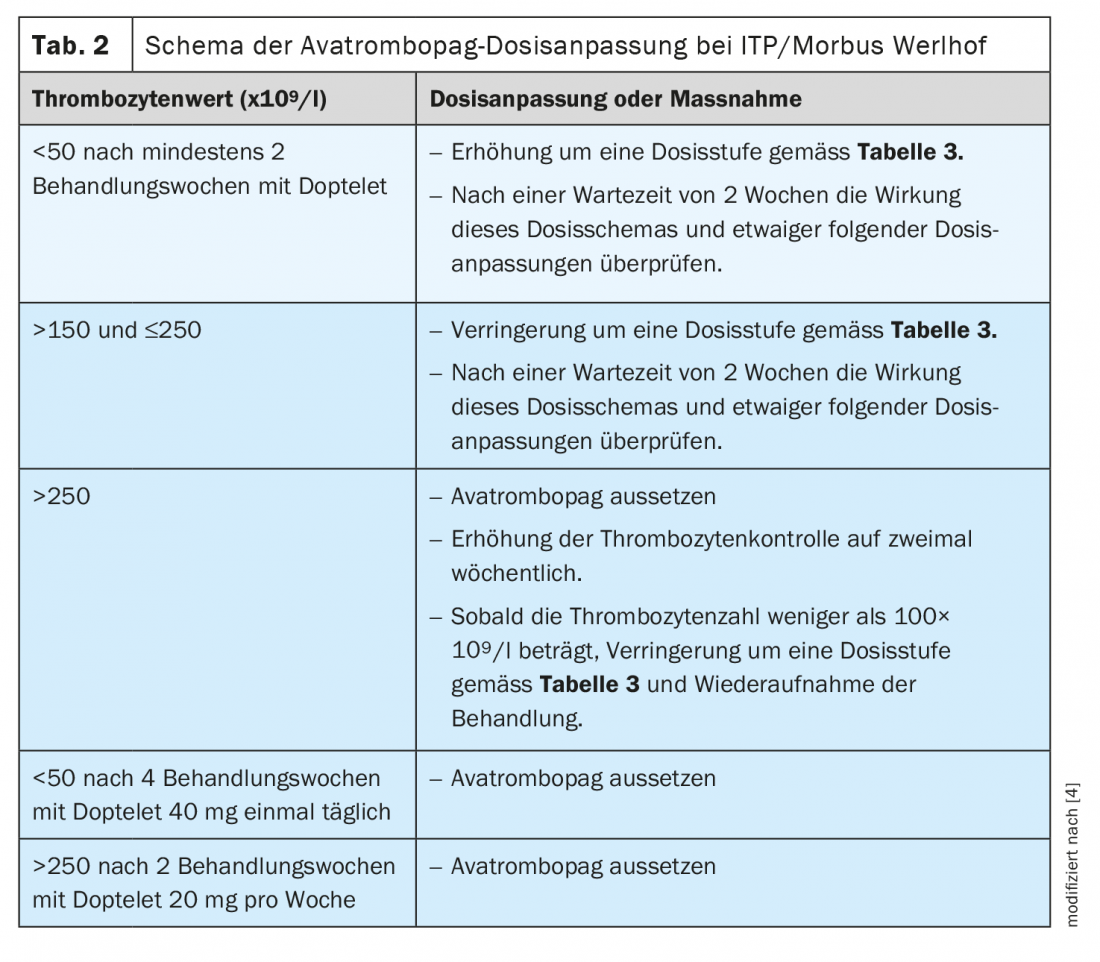
The indication for therapy in chronic immune thrombocytopenia is not solely dependent on the bleeding tendency and platelet count, but also on the stage of disease, course of disease and other individual factors. Corticosteroids are used for first-line therapy in adults. Different therapeutic methods are available as second-line treatment. In addition to splenectomy or immunosuppressants, thrombopoietin receptor agonists may be used [5]. The recently approved avatrombopag is a second-generation thrombopoietin receptor agonist. To reduce the risk of bleeding, use the lowest avatrombopag dose necessary to achieve and maintain a platelet count of ≥50 ×109/l [4]. In clinical trials, platelet counts generally increased within 1 week of starting treatment with avatrombopag and decreased within 1 to 2 weeks after discontinuation. The recommended starting dose is generally 20 mg (1 tablet) once daily with a meal. For patients taking moderate or potent dual inducers or moderate or potent dual inhibitors of CYP2C9 and CYP3A4/5 or of CYP2C9 , the starting dose must be adjusted. During the course of therapy, specific dose adjustments based on platelet count response apply to all Werlhof disease patients (Table 2, Table 3) [4].
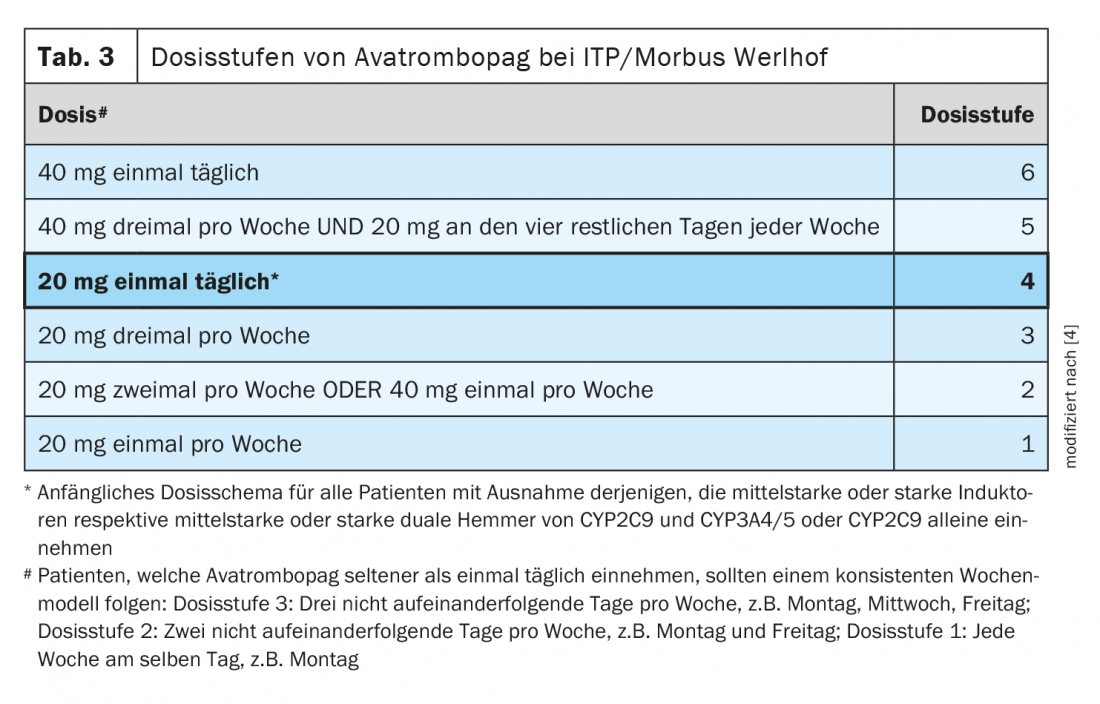
A daily dose of 40 mg avatrombopag (2 film-coated tablets) should not be exceeded. After initiation of therapy, the platelet count should be assessed at least once a week until a stable value between ≥50 ×109/l and ≤150 ×109/l has been achieved [4]. For patients receiving avatrombopag only once or twice weekly, check platelet counts twice weekly during the first weeks of therapy. Twice weekly monitoring is also required after dose adjustments during the course of therapy. Because of the potential risk for platelet levels above 400 ×109/l during the first weeks of therapy, patients should be carefully monitored for signs or symptoms of thrombocytosis [4]. After stable platelet values have been achieved, they should be checked at least once a month. After discontinuation of avatrombopag, platelet counts should be determined once weekly for at least 4 weeks.
Literature:
- Kuter D: MSD manual: professional version; overview of platelet disorders. 2020. www.msdmanuals.com (last call 03/21/2022)
- Neunert C, et al: Blood Adv 2019; 3(23): 3829-3866.
- Markham A: Avatrombopag: Drugs 2021: 81: 1905-1913.
- Drug Information, www.swissmedicinfo.ch (last accessed Mar. 21, 2022).
- “Immune Thrombocytopenia (ITP),” Onkopedia, www.onkopedia.com/de (last accessed Mar. 21, 2022).
- New registrations, www.swissmedic.ch (last accessed 03/21/2022)
HAUSARZT PRAXIS 2022; 17(4): 28-29
InFo ONCOLOGY & HEMATOLOGY 2022; 10(3): 30-31.