Once called the “disease of kings,” gout is now a common disease due to prosperity. The genetic predisposition for the metabolic disorder is inherited, but various conditions come together for an outbreak. For example, obesity and a high-fat, opulent diet significantly promote the onset. Prophylaxis therefore includes, in addition to nonpharmacological measures such as a healthy diet and plenty of exercise, the use of uricostatic drugs with the aim of reducing serum uric acid levels to below 360 μmol/l lower.
As a rule, excess uric acid is excreted via the kidneys and intestines. However, if the body forms too much uric acid or excretes too little, the concentration in the blood increases and crystals form, which are deposited in the tissues. With increasing age, this metabolic disorder occurs with increasing frequency. About 1-2% of the population suffers from it, with women being affected much less frequently than men. However, this difference decreases with the onset of menopause, since from this point on the favorable influence of estrogen on uric acid excretion ceases.
Factors that promote gout include a purine-rich diet and alcohol, as well as hypertriglyceridemia, obesity, hypertension, hypercholesterolemia, diabetes, renal insufficiency, and various medications, such as aspirin or diuretics, which must certainly be considered in a therapy [3]. In addition, gout is associated with many comorbidities. For example, gout patients have an increased risk of renal insufficiency, kidney stones, or cardiovascular disease.
The increase of the uric acid level
Uric acid is formed via purine metabolism. Purines ingested with food or produced during metabolism and cell decay are metabolized to uric acid. Essential steps here are the degradation of xanthine and hypoxanthine by the enzyme xanthine oxidase. The enzyme uricase is required to convert these into allantoin, which can be excreted in the urine. Primates and some reptiles, however, have lost the functionality of the uricase gene in the course of their evolution. Which is why 20% of uric acid is excreted through the intestine and 80% through the kidney, but a large part of it is reabsorbed in the tubules.
Gout does not develop overnight. According to Susanna Enderlin Steiger, MD, a specialist in rheumatology and internal medicine at RheumaClinic Bethanien in Zurich, it develops over a long period of time from elevated uric acid levels, known as hyperuricemia [1]. This preliminary stage of gout does not initially cause any physical symptoms, but it is an initial warning sign that is often discovered by chance during a blood test. As a result of the increase in uric acid, crystals known as urates form, which are deposited in the body preferentially in the joints and kidneys. As the buildup increases, a gout attack is inevitable. Without treatment, chronic gout then develops, in which the gout pain occurs in episodes, at increasingly shorter intervals.
Various clinical manifestations
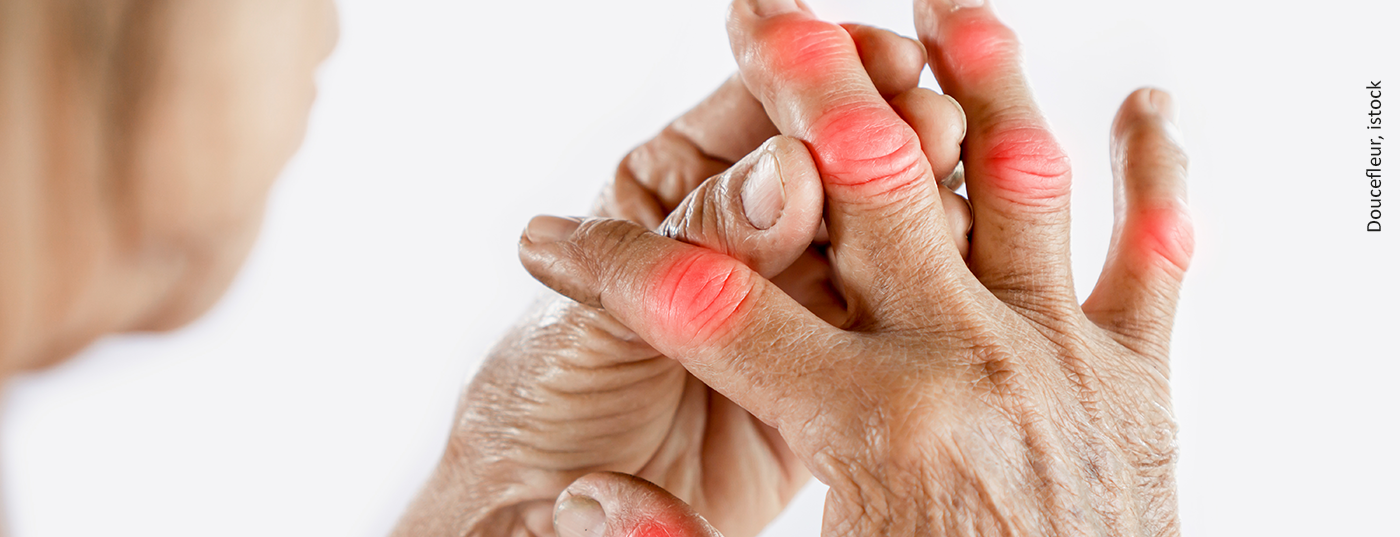
Gout can manifest itself clinically in different ways. In addition to an acute attack of gout and chronic gout, multilocular gout may also occur. Multilocular changes may be seen in different joints and, unlike the acute attack of gout, may lead to chronic arthritis and destruction. The characteristic symptoms of acute gout are usually clear. In addition to swelling and redness, there is usually sharp pain and severe functional limitation of the affected joint, often the big toes. X-rays reveal the long-term damage to the affected joints: joint destruction with sharply punched out usures at the ends of the bones, a reduced joint space and soft tissue changes, so-called gouty tophi, characterize gout that is usually already chronic.
The gold standard is still crystal detection in the synovial fluid or in the punctate of a tophus. If there is no possibility of a punctate, imaging techniques may also be diagnostically helpful. Utrat deposits can also be detected by ultrasound or dual-energy CT (DECT). The typical changes of the double contour or inhomogeneous tophi can also be detected here. Whereas the DECT has a sensitivity of 90% and a specificity of 80%. However, serum uric acid is not used to make a diagnosis, as it may be lowered during an acute attack of gout.
Therapy of the acute gout attack
EULAR recommends treating a gout flare as early as possible. Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly used drugs in this case. The group of anti-inflammatory painkillers includes, for example, diclofenac, ibuprofen, indometacin, naproxen and etoricoxib. However, they are contraindicated in relevantly impaired renal function; in mildly impaired renal function or moderate heart failure, they should be administered only when strictly indicated and for a very short time. Colchicine can also effectively relieve the acute attack of gout. One treatment consists of taking 1 mg, after two hours another 0.5 mg is administered once, in the following days 2× 0.5 mg/day until the symptoms subside. The well-tried autumn crocus alkaloid is still the drug of first choice for acute gout attacks, although it may only be used in strictly limited doses due to its toxic effects. Failure to do so can cause violent diarrhea that can be life-threatening. In addition, bleeding and heart failure may occur. A recent study showed that the anti-inflammatory effects of colchicine reduced the risk of cardiovascular events in patients with recent myocardial infarction. However, evidence of such risk reduction in patients with chronic coronary disease is limited [4].
Peroral administration of steroids has also been established in the treatment of acute gout attack. Up to 30-35 mg can be administered over five days. Also well possible is a combination of the previously mentioned drugs. In acute gout attacks, the interleukin-1 (IL-1) inhibitors anakinra and canakinumab are also recommended, although anakinra is not approved in Switzerland. Both IL-1 inhibitors act rapidly and safely on the direct inflammatory mechanism in the gout attack, but are not covered by health insurance. One treatment consists of 100 mg for 3 days.
Anti-inflammatory prophylaxis of further gout attacks.
After the acute phase, the long-term treatment of gout aims to normalize and maintain stable uric acid levels and prevent repeated gout attacks and further progression of the disease. However, in the first weeks to months after starting uric acid-lowering therapy, gout attacks may occur more frequently. For prophylaxis of further gout flares, a low-dose NSAID, low-dose steroids 5mg, or colchicine 2 x 0.5 mg/day may be prescribed for 6 months. To keep the risk of gout attacks as low as possible, it is recommended that the uric acid-lowering therapy be phased in.
Reduction of uric acid concentration in serum
Uric acid-lowering drug therapy is indicated for more than two attacks per year, tophous gout, renal insufficiency, joint destruction, or urate stones. The aim is to reduce serum uric acid levels to below 360 μmol/l or, in the case of tophi, to below 300 μmol/l. In drug therapy, there is the possibility of inhibiting xanthine oxidase, which prevents the formation of uric acid, on the one hand, and the use of probenecid, which inhibits the reabsorption of uric acid in the kidney, on the other. A rather rarely used method is to administer the missing enzyme uricase as a drug, which would convert uric acid into allantoin. However, since uricase is not tolerated by most people, it is only used when the usual drugs in the highest permitted dosage do not have a sufficiently strong effect or are not tolerated.
The xanthine oxidase inhibitor allopurinol is considered the first-choice basic drug therapy. Therapy should begin after an episode has healed, because the medicinal influence on the uric acid level can lead to a new episode. If creatinine is normal, start with a starting dose of 50-100 mg/day. This is followed by a gradual increase every three to four weeks until the target value of a maximum of 600-800 mg/day is reached. In case of existing renal insufficiency, the dosage must be adjusted. In rare cases, Lyell syndrome may occur with allopurinol. The risk is higher with higher starting doses, existing renal insufficiency and concomitant diuretic therapy, and in elderly or female patients, as well as the genetic marker HLA B5801. As an alternative to allopurinol, the xanthine oxidase inhibitor febuxostat can be used. The starting dose is 40 mg/day, increasing to 80 mg/day after four weeks. Contraindications here are severe liver insufficiencies and renal insufficiencies.
Only probenecid is currently available as a uricosuric. The dosage is 250-1000 mg 2× daily until normalization of serum uric acid levels, followed by gradual reduction of the dose. Probenecid may be taken in addition to xanthine oxidase inhibitors. Again, use the uricosuric only in renally healthy patients. Only in exceptional cases, in the case of uncontrollable gout or in tumor patients, is treatment with uricolytics such as pegloticase or rasburicase carried out. Possible concomitant therapies with uricosuric effects include losartone, amlodipine, and fenofibrate. If possible, treatment with diuretics should be stopped.
Avoid foods rich in purines
As a non-medicinal measure, a gout diet should be followed. Gout patients should avoid purine-containing foods and there should be a general shift from meat and fish to consuming more vegetables and dairy products. In addition, sufficient fluids should be consumed, but this should not include drinks with a high fructose content, such as fruit juices and sweet drinks. In addition, alcohol should be avoided, especially beer.
Take-Home Messages
- Force diagnosis: Punctate, ultrasound, dual-energy CT
- Therapy of the seizure: NSAIDs, colchicine, steroids, (IL-1) inhibitors.
- Uric acid-lowering therapy from the first episode: xanthine oxidase inhibitors, uricosurica
- Treat to target: uric acid level <360 μmol/l or <300 μmol/l
- Treat concomitant diseases: Obesity, lipids, arterial hypertension, diabetes, etc.
Congress: FomF 2021
Literature:
- Enderlin Steiger S: Gout an update. General Internal Medicine Update Refresher, Forum for Continuing Medical Education, Aug. 21, 2021.
- Dörner K: Clinical Chemistry and Hematology (ISBN 9783131297181), 2013 Georg Thieme Verlag.
- Chen JH: Severity of different metabolic risk impact on gout incidence. Eularp op-0290.
- Nidorf SM, et al: Colchicine in patients with chronic coronary disease. N Engl J Med 2020; doi: 10.1056/NEJMoa2021372.
HAUSARZT PRAXIS 2021; 16(9): 36-37 (published 9/19-21, ahead of print).