The current EULAR guidelines recommend anti-B cell therapy with belimumab as an additive to standard therapy in systemic lupus erythematosus. Recently published trial data now show that belimumab also has proven clinical benefit in lupus nephritis. This is highly relevant because inflammation of the kidneys is a dreaded manifestation of a lupus flare and current treatment options are inadequate.
Systemic lupus erythematosus (SLE) is a chronic inflammatory, usually relapsing autoimmune disease. The clinical manifestations are characterized by a high heterogeneity and may affect different organs (skin, lungs, heart, CNS, muscles/joints, kidneys). In the past two years, important innovations have occurred in the classification and treatment of SLE. According to the EULAR/ACR classification 2019 [1], a positive ANA result is mandatory for the diagnosis of SLE, and a patient must also score ≥10 points on the corresponding scale. Clinical manifestations and immunologic criteria are weighted differently and apply only if not better explained by other causes. With a sensitivity of 96.1% and a specificity of 93.4%, this diagnostic system is very informative.
Modern Management of SLE: Treat-to-target Approach
In recent decades, increasing understanding of immunologic pathomechanisms has led to advances in therapy, significantly improving life expectancy in SLE patients. In addition to ensuring long-term survival, the goal is to reduce organ damage and improve quality of life. This requires consistent therapy and regular evaluations of disease activity. In the update of the EULAR recommendations [2], hydroxychloroquine is recommended in all SLE patients, with a maximum dosage of 5 mg/kg body weight. During maintenance therapy, glucocorticoid use should be limited to less than 7.5 mg/day (prednisone equivalent), and if possible, avoided altogether. Early use of the immunosuppressive agents methotrexate, azathioprine, and mycophenolate is advised. With the biologics, new substance classes have found their way into the recommendations. To date, Belimumab (Benlysta®) [3] is the only monoclonal antibody approved for the indication SLE, off-label sometimes Rituximab is also used.
EULAR recommends belimumab as add-on treatment
An update of the EULAR recommendations on the management of SLE reinforces the therapeutic value of belimumab in SLE [2]. “There is a high level of evidence that belimumab should be considered as an add-on treatment in patients with inadequate response to standard therapy,” summarized Prof. Andreas Schwarting, MD, Johannes Gutenberg University, Mainz, Germany, at the DGIM Annual Meeting. Belimumab is a monoclonal antibody directed against B-cell activation factor, which has selective immunosuppressive properties. Binding to the soluble human B lymphocyte stimulator protein BLyS (“soluble human B lymphocyte stimulator protein”) shortens the lifespan of CD20+ B lymphocytes and plasma cells. Elevated BLyS levels are a pathophysiological finding in patients with systemic lupus erythematosus and other autoimmune diseases.
BLISS-LN study: Belimumab also effective in lupus nephritis
High dsDNA antibody titers are considered an important predictor for the development of lupus nephritis. “Data from a long-term open-label study show that anti-dsDNA antibody titers can be reduced by up to 90% with belimumab.” [4,5]. Lupus nephritis is an inflammation of the kidneys caused by SLE, which occurs in 25-60% of SLE patients [6]. There is a need for improved therapeutic options. Renal involvement is among the most severe organ manifestations with often life-threatening courses. In 10-30% of patients with lupus nephritis, renal disease requiring dialysis develops despite standard treatment. A phase III study published in 2020 (BLISS-LN) suggests that BLyS/BAFF blockade with belimumab also helps in lupus nephritis. Belimumab has been shown to significantly improve recovery of renal function and mortality in addition to classical lupus therapy with immunosuppressants (mycophenolate/cyclophosphamide/azathioprine mostly with glucorticoids).
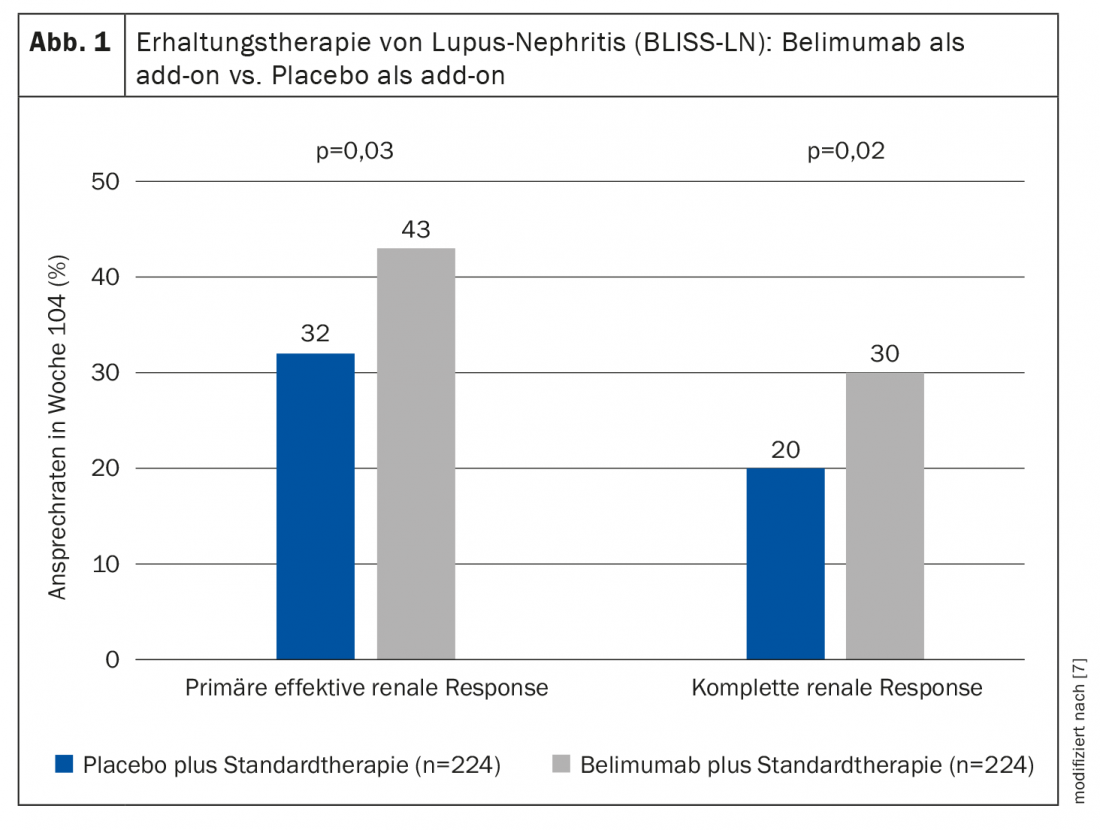
In the double-blind, placebo-controlled multicenter study, adults with biopsy-proven active lupus nephritis were randomized to the belumimab study arm (n=224) or placebo (n=224) in addition to standard therapy [7]. Patients in the experimental condition received intravenous belimumab at a dose of 10 mg per kg body weight as an add-on to standard treatment. The primary end point was effective renal response, defined as improvement in urinary protein excretion (urinary protein:creatinine ≤0.7) and renal filtration rate (glomerular filtration rate/eGFR ≥60 ml/min/1.73 m2 or decrease in eGFR* of less than 20% of prenephritis eGFR). At week 104, significantly more patients in the belimumab group met the primary endpoint (43% vs. 32%; odds ratio, 1.6; 95% confidence interval [KI], 1.0 to 2.3; p=0.03) (Fig. 1). The main secondary endpoint, complete renal remission was also achieved (30% vs. 20%; odds ratio, 1.7; 95% CI, 1.1 to 2.7; p=0.02). The risk of renal-related event or death was lower in patients receiving belimumab than in patients receiving placebo (hazard ratio, 0.51; 95% CI, 0.34 to 0.77; p=0.001). The safety profile of belimumab was consistent with that of previous studies.
Congress: DGIM Annual Conference 2021
Literature:
- Aringer M, et al: 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis & Rheumatology 2019; 71(9): 1400-1412.
- Fanouriakis A, et al: 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736-745.
- Swissmedicinfo: Medicinal product information, www.swissmedicinfo.ch (last accessed 09.06.2021).
- Schwarting A: SLE and connective tissue disease. Prof. Dr. med. Andreas Schwarting, DGIM Annual Meeting, 19.04.2021
- Wallace DJ, et al: Safety and efficacy of belimumab plus standard therapy for up to 13 years in patients with systemic lupus erythematosus. Arthritis Rheum 2019
- Hanly JG, et al: The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016; 55: 252-262.
- Furie R, et al: Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N Engl J Med 2020; 383: 1117-1128.
HAUSARZT PRAXIS 2021; 16(7): 30-32 (published 6/28/21, ahead of print).