Even in the era of VEGF and checkpoint inhibitors, the prognosis for advanced renal cell carcinoma remains poor. The use of targeted therapies and immunotherapeutic approaches is difficult due to lack of clear targets and low immunogenicity. Nevertheless, there are some new treatment concepts that may shape the management of this disease in the coming years and raise faint hope for the future.
Currently, the treatment of metastatic renal cell carcinoma focuses in particular on checkpoint and VEGF (vascular endothelial growth factor) inhibitors. Thus, anti-VEGF/immunotherapy combinations such as axitinib/pembrolizumab or the immunotherapy combination nivolumab/ipilimumab are mostly used in the first line. While the use of these substances has improved the prognosis, there is still room for improvement. With the development of new therapeutic approaches such as hypoxia-inducible factor 2α (HIF-2α) blockade, important steps are currently being taken that may contribute to more successful management in the near future. In addition to inhibition of HIF-2α, the focus is on the use of modified interleukin-2(IL-2) and antibody-drug conjugates and therapeutic concepts that interfere with the metabolism of malignant cells.
A look back
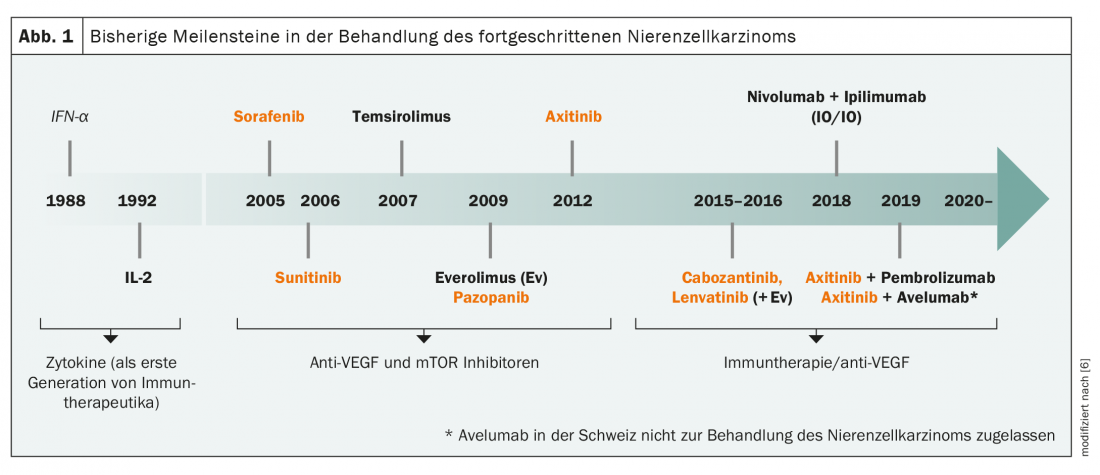
The history of immunotherapy in renal cell carcinoma goes back to the 1980s, when cytokines such as interferon-α and interleukin-2 were used as an early form of immunotherapy (Fig. 1) . Then it was quiet for a long time about new therapeutic options until 2005, when sorafenib became the first VEGF inhibitor on the market. With sunitinib, axitinib and pazopanib, further representatives of this drug class quickly followed, complemented by mTOR inhibitors such as everolimus. Finally, in the 2010s, the second era of immunotherapy for advanced renal cell carcinoma began with the checkpoint inhibitors pembrolizumab, nivolumab, and ipilimumab, so that today a large number of agents in various combinations are approved for its treatment. Nevertheless, the low immunogenicity of most tumors and the lack of clear targets lead to difficulties in the use of different immunotherapeutic approaches and targeted therapies. Few cases exist in which microsatellite instability or increased tumor mutational burden (TMB), both predictive markers for immunotherapeutic approaches, can be detected. There is also a lack of known driver mutations that are suitable therapeutic targets. Nevertheless, several clinical trials of targeted therapies are ongoing, including antibody-drug conjugates and the MET protooncogene.
Targeted therapies: Antibody-drug conjugates and MET protooncogene.
The MET protooncogene could gain importance in the future, especially in the treatment of the rare papillary renal cell carcinoma. However, monotherapy with savolitinib as well as crizotinib has not been convincing in previous studies. Currently, the compounds are being tested in combination treatments.
Current developments also suggest that caution is called for with regard to antibody-drug conjugates. These drugs consist of three components: An antibody with high specificity for tumor-associated antigens, a linker that is stable in the bloodstream but releases the drug in the target cell, and the drug itself. Three studies on such antibody-drug conjugates had to be discontinued in the recent past due to lack of efficacy. In contrast, two compounds that are still being investigated in clinical trials are BA-3021 and DS-6000a. The latter drug has the active ingredient deruxtecan, which has already been used successfully in breast carcinoma.
HIF-2α inhibition promising
More encouraging and already somewhat more mature are the data on hypoxia-inducible factor 2α (HIF-2α) blockade. This accumulates in those affected who have a mutation of the von Hippel-Lindau tumor suppressor gene– and this is after all 90% of all patients suffering from clear cell renal cell carcinoma. The overproduction of HIF-2α creates a deceptive signal that signals oxygen scarcity, leading to an increase in blood supply and more rapid tumor growth.
The pathogenetic mechanism triggered by HIF-2α can be interrupted by the selective small-molecule inhibitor belzutifan. This is currently being diligently investigated in clinical trials (Tab. 1) . So far, the substance has shown promise both as monotherapy and as part of combination treatments. For example, in a phase I/II trial with 55 participants, a reduction in tumor size was observed in 64%, and the objective response rate was 25%. It must be taken into account that this was a heavily pretreated study population, with a median of three lines of therapy already completed. Response duration exceeded six months in 71% of participants, and 19 of the 55 patients continued treatment after one year [1].
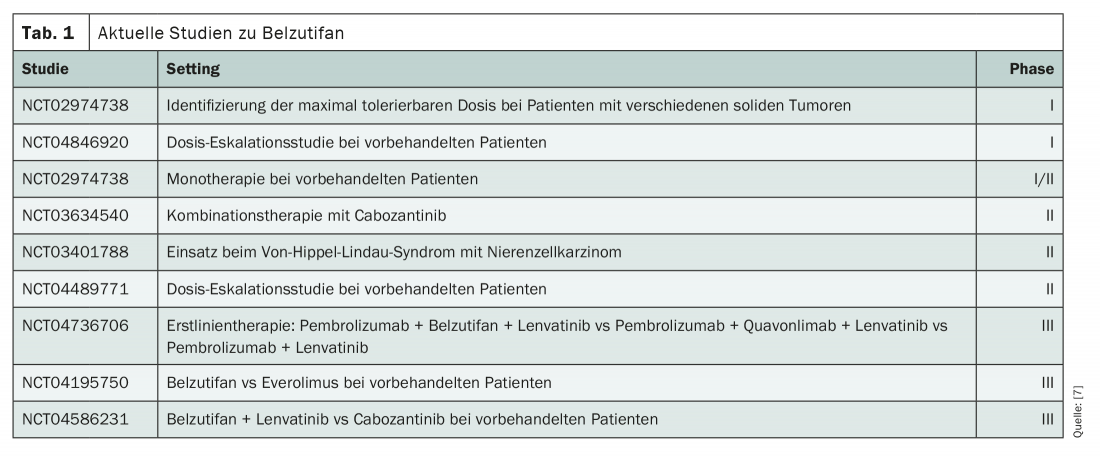
In combination with cabozantinib, similar results were seen in a recently presented phase II study [2]. Thus, 88% of the total 41 pretreated patients experienced a reduction in their tumor size, the objective response rate was 22%, and the median progression-free survival (PFS) was 16.8 months. After one year, 81% of the patients were still alive. Phase III trials are investigating the first-line use of Belzutifan itself. Here, the compound is being tested in combination with pembrolizumab and lenvatinib. In later lines of therapy, belzutifan is currently on trial as monotherapy against everolimus and in combination with lenvatinib against cabozantinib. So far, dose-limiting factors seem to be especially the anemia induced by Belzutifan, which is due to a reduction of erythropoietin.
Targeting tumor metabolism
In addition to HIF-2α, the metabolism of malignant cells also offers a potential new target. Thus, overactivation of tumor cell growth leads to abnormal glucose metabolism. This phenomenon, known as the “Warburg effect,” leads to a lack of metabolites and increased lactate formation. Nevertheless, to ensure an adequate supply, malignant cells ramp up their glutamine metabolism. This can be disrupted by the drug telaglenastat, which is currently being tested in combination with cabozantinib in a phase I trial [3]. To date, the compound has shown encouraging activity with ongoing responses in advanced cases.
In addition, telaglenastat is being studied in the Phase II CANTATA trial, which is evaluating the addition of the compound to cabozantinib in the second and third lines of therapy. Included are 444 patients without prior cabozantinib treatment, with completion expected in 2022. Unfortunately, in an analysis published in January 2021, the primary endpoint of PFS was not met. However, if the safety profile is good, the trial will continue, particularly to test telaglenastat in other entities such as non-small cell lung cancer.
Back to the Roots: Interleukin-2
Interleukin-2 (IL-2) is currently experiencing a rebirth as a potential therapeutic option in renal cell carcinoma. Even the original studies on aldesleukin from the 1980s often showed an impressively long duration of response. However, response rates to treatment were low and the compound proved toxic. The death rate was 4%, which was particularly due to capillary leak syndrome triggered by IL-2 [4]. Various modifications of IL-2 should now prevent these negative effects and increase the response rate. For example, α-chain binding can be prevented, resulting in fewer side effects and increased proliferation of effector T cells while producing fewer regulatory T cells.
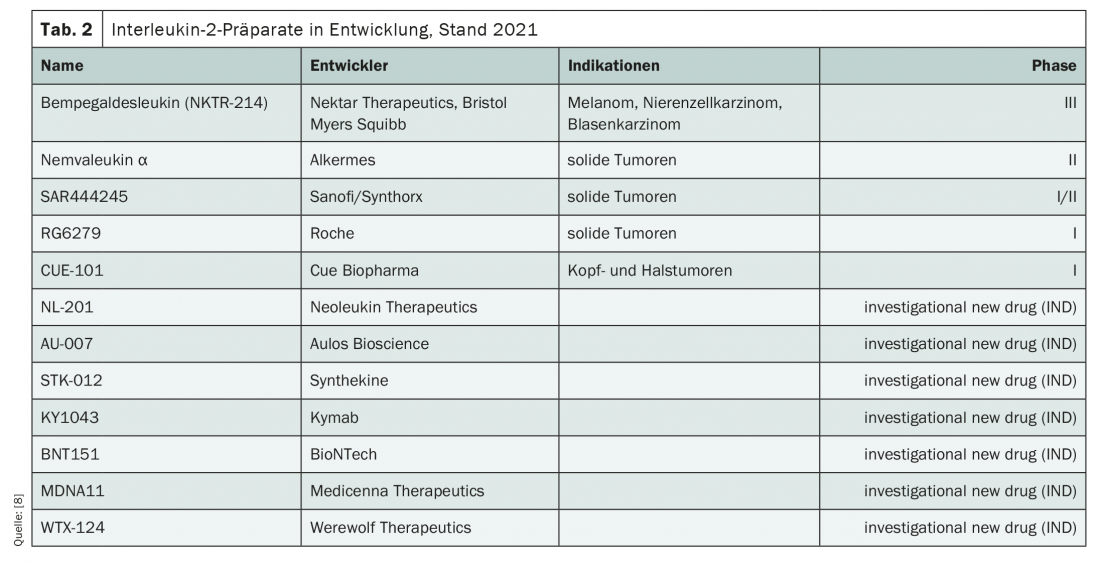
Currently, a colorful bouquet of IL-2 candidates from various companies is in development (Tab. 2) . Bempegaldesleukin, which is currently being investigated in the phase III PIVOT-09 trial, has the most data. It is to be used in melanoma, renal cell carcinoma as well as bladder cancer. In combination with nivolumab, the response rate in early studies in a phase I trial was 71.4% in the first line of therapy, compared to 28.6% in the second line [5]. Other examples of chemical IL-2 modifications represent nemvaleukin α and SAR444245, which are being evaluated in phase II trials for use in solid tumors.
Quintessence
Although longer-term data on the use of HIF2α inhibitors, IL-2, antibody-drug conjugates, and inhibitors of glutamine metabolism in clear cell renal cell carcinoma remain to be seen, and there are still some hurdles to overcome before broad clinical application, something is happening in the pipeline. In particular, blockade of HIF2α has shown promise in clinical trials. However, these successes cannot hide the fact that specific options are still lacking, especially for papillary, chromophobe and medullary subtypes according to , and appropriate targets need to be identified.
Source: presentation “Novel Targets and Promising New Therapies for Renal Cancer,” Howard Burries, American Association for Cancer Research (AACR) annual meeting, Apr. 09-14, 2021, virtual conduct.
Literature:
- Bauer TM: The oral HIF-2 α inhibitor MK-6482 in patients with advanced clear cell renal cell carcinoma (RCC): Updated follow-up of a phase I/II study2021; Presentation, American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium, Feb. 11-13, 2021.
- Choueiri TK: Phase 2 study of the oral hypoxia-inducible factor 2α (HIF-2α) inhibitor MK-6482 in combination with cabozantinib in patients with advanced clear cell renal cell carcinoma (ccRCC); presentation, American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium, Feb. 11-13, 2021.
- Meric-Bernstam F, et al: CB-839, a glutaminase inhibitor, in combination with cabozantinib in patients with clear cell and papillary metastatic renal cell cancer (mRCC): Results of a phase I study. Journal of Clinical Oncology. 2019; 37(7_suppl): 549.
- Fyfe G, et al: Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995; 13(3): 688-696.
- Diab A, et al: Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020; 10(8): 1158-1173.
- Choueiri T: Advanced Kidney Cancer Update – “A Glimpse into the Future”. Presentation, SOCIETY OF UROLOGIC ONCOLOGY (SUO) 21ST ANNUAL MEETING; Washington, DC, December 2020.
- www.clinicaltrials.gov (last accessed 04/16/2021)
- Mullard A: Restoring IL-2 to its cancer immunotherapy glory. Nat Rev Drug Discov. 2021; 20(3): 163-165.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(3): 18-21 (published 6/17/21, ahead of print).