Immunotherapy is also becoming increasingly important in the treatment of urothelial carcinoma. Checkpoint inhibitors are already being used successfully, particularly in advanced tumors. Now, potential applications of avelumab, atezolizumab and co. are already emerging in earlier disease stages and lines of therapy.
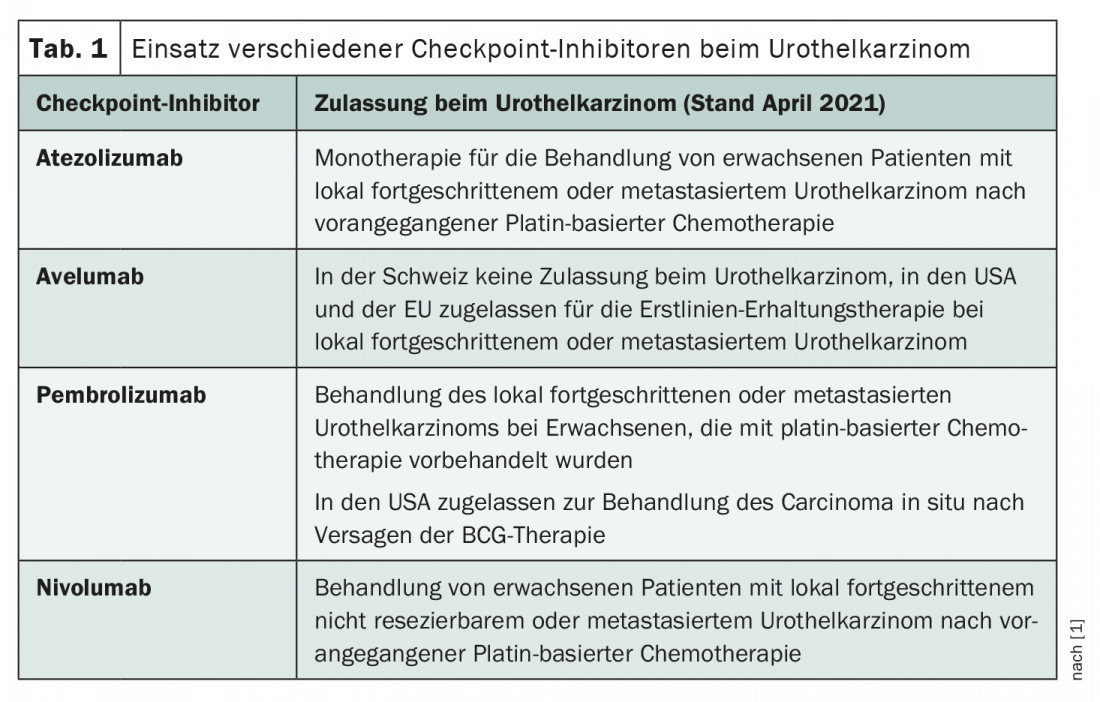
In Switzerland, checkpoint inhibitor therapy for urothelial carcinoma is currently approved exclusively for second-line treatment of locally advanced or metastatic tumors [1]. However, the recent extensions of indications for avelumab and pembrolizumab by the EMA (European Medicines Agency) and FDA (U.S. Food and Drug Administration) are likely to herald a broader use of these agents in the future. Arlene O. Siefker-Radtke, professor of urogenital oncology at the University of Texas, took a closer look at the role of checkpoint inhibitors in urothelial carcinoma at this year’s NCCN Annual Meeting, presenting an overview of the hottest research questions in the field.
Established standard in advanced tumors
Over the years, platinum-based chemotherapies in particular have proven effective in the treatment of advanced urothelial carcinoma. Thus, gemcitabine/cisplatin and MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) remain the most common first-line treatments today [2]. However, already at the beginning of the millennium there were efforts to explore immunotherapeutic options. For example, attempts have been made to use interferon-α2b [3]. However, these remained without significant success – until the checkpoint inhibitors came along. In the second line, immunotherapy rapidly gained acceptance and Swissmedic approved atezolizumab, nivolumab and pembrolizumab for this indication. Further areas of application are currently being intensively researched, and the entry of checkpoint inhibitors into first-line therapy is not only possible but likely in the near future. Indeed, it was not until January of this year that avelumab received approval in Europe for first-line maintenance therapy in advanced urothelial carcinoma, less than a year after the FDA gave the green light (Table 1) .
In advanced urothelial carcinoma, Siefker-Radtke said, there are basically three treatment approaches involving checkpoint inhibitors that are currently in focus. In her presentation, she distinguished between combination therapies, maintenance therapies, and sequential use.
Checkpoint inhibitor yes – but how?
In addition to combinations of chemotherapy and checkpoint inhibitors, immunotherapeutics are currently also being tested in maintenance therapy after response to chemotherapy and in sequential use – i.e. in disease progression after first-line therapy. According to Siefker-Radtke, the optimal timing of the deployment in particular is still unclear. He said that the most convincing data exist on the benefits in the second line. For example, pembrolizumab prolongs median overall survival by approximately three months after disease progression compared with taxane-based chemotherapy [4]. The checkpoint inhibitor also scores with higher response rates and a more favorable toxicity profile. It is therefore not surprising that checkpoint inhibitors are already approved for this indication in Switzerland.
Data on the benefit of maintenance therapy after response to first-line chemotherapy are somewhat thinner. Avelumab in particular is currently the focus of attention in this area. The compound was approved for maintenance therapy by the FDA in 2020 and by the EMA in January of this year. The latest data were presented at the ASCO (American Society of Clinical Oncology) Congress 2020 and raise hope that avelumab could soon play a significant role as a first-line drug in Switzerland as well – with at least a slight improvement in prognosis in advanced urothelial carcinoma. Thus, the median overall survival in the study was prolonged by maintenance therapy from 14.3 to 21.4 months. However, Siefker-Radtke emphasized, there was also less clear data available. For example, pembrolizumab has not been convincing in maintenance therapy to date. Improvement in progression-free survival (PFS) but no prolongation of overall survival was observed [5]. The expert attributed this, among other things, to the fact that the study on pembrolizumab allowed a cross-over, i.e. the use of the immunotherapeutic in the case of progression, while that on avelumab excluded this option. Siefker-Radtke concluded that the timing of application is potentially not that critical and the use of checkpoint inhibitors per se may be the determining factor. Or to put it another way, “It’s a question of EVER immunotherapy vs. NEVER immunotherapy.” In this regard, it may well make sense to forgo early use and wait for disease progression or recurrence before administering immunotherapy. Finally, one should not forget the side effects. Thus, whether maintenance therapy with checkpoint inhibitors will become widespread remains to be seen.
Another focus of various studies is the exploration of a parallel use of chemotherapy and immunotherapy in the first line. Promising data were presented at the 2018 European Society of Medical Oncology (ESMO) Congress on the neoadjuvant administration of a combination therapy of gemcitabine and pembrolizumab, and the IMvigor130 trial is investigating the addition of atezolizumab to chemotherapy in advanced urothelial carcinoma, to name two examples. To date, this approach has not been approved anywhere, but with the results of larger studies, changes in treatment are potentially on the horizon in the next few years. In her presentation, Siefker-Radtke discussed reasons for and against combination therapy.
Tension between synergism and antagonism
The effect of simultaneous administration of chemotherapy and immunotherapy is not easy to assess for several reasons. The manifold influences on the immune system and various interactions lead to an extremely complex situation. For example, while chemotherapy leads to increased presentation of antigens and thus could enhance the effect of checkpoint inhibitors, it also causes immunosuppression.
Siefker-Radtke highlighted two reasons in particular for simultaneous therapy. In addition to increased antigen presentation by chemotherapy, which may be beneficial for immunotherapy, checkpoint blockade is conversely potentially beneficial for chemotherapy efficacy, he said. For example, increased PD-L1 expression after chemotherapy is a prognostically unfavorable factor and could possibly be reduced by a targeted attack. Thus, the additional PD1/PD-L1 blockade could help prevent chemotherapy resistance [6,7]. Studies to date suggest that combination therapy may be particularly worthwhile in sufferers with liver metastases. This subgroup with usually rapidly progressive disease has a significantly poorer response to monotherapy with checkpoint inhibitors, as shown in studies of cisplatin-naïve patients [8,9].
However, there are also considerations that argue against the joint use of chemotherapy and immunotherapy agents. Thus, the effect of checkpoint inhibitors could be attenuated by chemotherapy-induced neutropenia [10]. Also, according to Siefker-Radtke, various other mechanisms occur in the course of chemotherapy that promote tolerance of the immune system and thus counteract immunotherapy. For example, the number of dendritic cells decreases under chemotherapy, while the number of regulatory T cells increases. In this area of conflict between synergistic and antagonistic effects, results of larger studies remain to be seen before the actual benefit of combination therapy can be conclusively assessed.
Checkpoint inhibitors in earlier disease stages.
There is increasing data supporting the use of checkpoint inhibitors in earlier stages of disease as well. Here, the expert presented two indications in which immunotherapy could soon be used: Superficial carcinoma in situ of the urinary bladder and as adjuvant therapy after radical surgery. In case of BCG treatment failure, pembrolizumab is already approved in the U.S. for the treatment of carcinoma in situ. In the Keynote 057 trial, the latest results of which were presented at ASCO Congress 2020, 40% of patients experienced a complete response to pembrolizumab at three months, which lasted an average of 16.2 months.
In addition, especially in cases of PD-L1 expression, adjuvant administration of checkpoint inhibitors raises hope. For example, postoperative administration of nivolumab for one year was studied in high-risk patients in the phase III Checkmate 274 trial. Recently published data show a prolongation of median disease-free survival in the overall population from 10.9 months on placebo to 21 months. This effect was even more impressive with PD-L1 expression ≥1%. In particular, a plateau emerged in the subgroup with PD-L1 expression – an indication of longer-term disease control, according to Siefker-Radtke.
So the bottom line is that there is a lot going on in urothelial carcinoma. In the coming years, checkpoint inhibitors may be increasingly used in earlier stages of disease – particularly adjuvant and carcinoma in situ. Whether its use as maintenance therapy in advanced urothelial carcinoma will become widespread remains to be seen. Combinations of chemotherapy and immunotherapy are currently in their infancy, but are by no means unthinkable.
Source: presentation “New Settings for Immune Checkpoint Inhibitors in Urothelial Bladder Cancer,” Arlene O. Siefker-Radtke, MD, at the NCCN Annual Meeting, Virtual Conduct March 18-20, 2021.
Congress: NCCN Annual Meeting
Literature:
- Medicinal product information from swissmedic. www.swissmedicinfo.ch (last accessed 28.03.2021).
- de Wit M, et al: Bladder carcinoma (urothelial carcinoma) – Onkopedia. Accessed March 2019. www.onkopedia.com/de/onkopedia/guidelines/blasenkarzinom-urothelkarzinom (last accessed Mar. 28, 2021).
- Siefker-Radtke AO, et al: Phase III trial of fluorouracil, interferon alpha-2b, and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in metastatic or unresectable urothelial cancer. J Clin Oncol. 2002; 20(5): 1361-1367.
- Bellmunt J, et al: Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017; 376(11): 1015-1026.
- Galsky MD, et al: Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients With Metastatic Urothelial Cancer. J Clin Oncol. 2020; 38(16): 1797-1806.
- Shin J, et al: Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Res Treat. 2019; 51(3): 1086-1097.
- Jiang Q, et al: CD19. Cancer Immunol Immunother. 2019; 68(1): 45-56.
- Balar AV, et al: Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017; 389(10064): 67-76.
- Balar AV, et al: First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017; 18(11): 1483-1492.
- Galsky MD, et al: Phase 2 Trial of Gemcitabine, Cisplatin, plus Ipilimumab in Patients with Metastatic Urothelial Cancer and Impact of DNA Damage Response Gene Mutations on Outcomes. Eur Urol. 2018; 73(5): 751-759.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(2): 28-29 (published 11/4/21, ahead of print).











