Iron deficiency in cardio-renal patients is not a trivial matter. Chronic heart failure, for example, is not only a common condition but also often associated with iron deficiency. This in turn has an impact on the forecast. This is because hospitalization and mortality rates are closely associated with the iron status of these patients. Early and guideline-based iron substitution is indicated.
Heart failure (HI) is primarily a disease of old age and the most common disease-related reason for hospitalization. Functional limitations of the heart are often accompanied by reduced kidney function – and vice versa. The reason is the close connection via both the sympathetic nervous system and the renin-angiotensin-aldosterone system. The latter controls, for example, the body’s fluid and electrolyte balance and thus also influences blood pressure. Effective arterial blood volume, in turn, is relevant to renal potassium loss. Both chronic renal and heart failure are also closely associated with iron deficiency, which in turn is an unfavorable prognostic factor in terms of exercise capacity, hospitalization, and mortality.
Pathophysiology of iron deficiency
The pathophysiology of iron deficiency in heart failure is likely multifactorial. Therefore, it is important not to overlook other causes such as gastrointestinal ulcers or malignant diseases. There may be simple factors, such as blood loss due to antiplatelet or anticoagulation therapy, that lead to iron loss. Malabsorption may also play a role. In addition, interstitial intestinal edema may result in decreased oral iron absorption.
The chronic inflammatory state associated with heart failure results in elevated levels of pro-inflammatory cytokines such as interleukin-6 (IL-6). Inflammation induces the synthesis of hepcidin, reducing the release of stored iron. While most chronic inflammatory diseases are associated with higher hepcidin levels, studies in HI patients have shown that advanced HI is associated with lower hepcidin levels and does not appear to correlate with IL-6 in this patient group. This may be due in part to increased erythropoietin levels associated with advanced HI, as well as hepicidin suppression.
Iron deficiency is widely underestimated, although it can be detected in almost every second patient. However, iron is essential for cellular respiration and physical performance. In addition, iron deficiency has been shown to directly affect human cardiomyocyte function, impairing mitochondrial respiration and reducing contractility and relaxation. This should therefore always be balanced early.
Substitute early
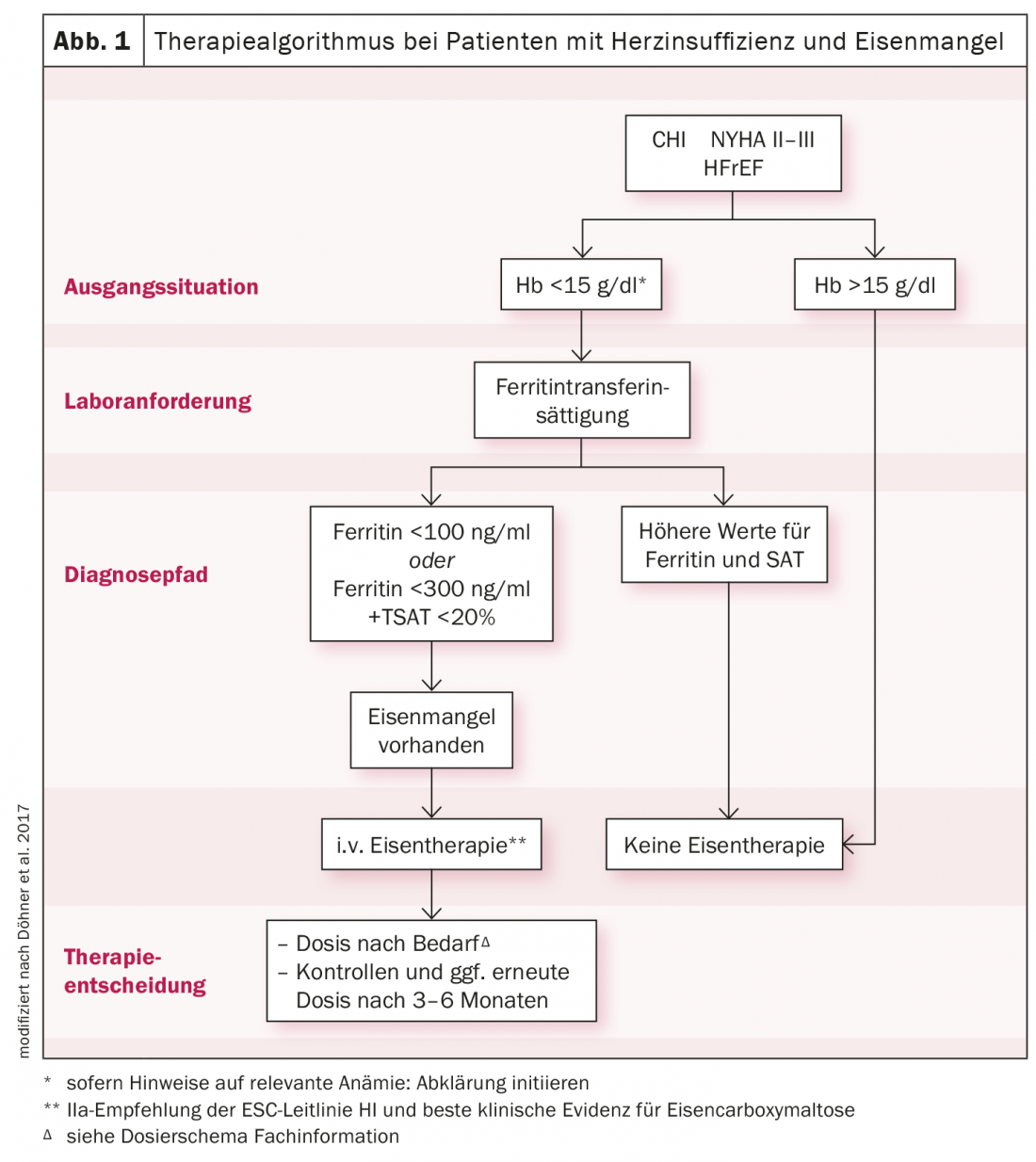
A blood count with ferritin, transferrin saturation (TSAT), CRP, and glomerular filtration rate should be used for basic diagnostics. In a patient with heart failure, serum ferritin levels <100 μg/l, or levels between 100-300 μg/l and TSAT <20% already indicate iron deficiency requiring treatment. Since oral iron substitution must be given over several months, is only absorbed to a reduced extent enterally, and has no effect on physical performance, the ESC guidelines suggest that i.v. administration of iron carboxymaltose should be considered (Fig. 1). In this way, iron stores can be replenished effectively, efficiently and in a controlled manner over a short period of time. Symptomatology is improved and hospitalization and mortality rates are reduced. A similar picture is seen in the cardio-renal patient. In stage III-IV, iron deficiency can be diagnosed in up to 70%. One study demonstrated a significantly faster replenishment of iron stores and a greater increase in TSAT levels with ferric carboxymaltose than with oral iron.
Ferric carboxymaltose fast effective
Ferric carboxymaltose exhibits high complex stability, making it applicable at high doses (up to 1000 mg/application and week) and in a short time (1000 mg in at least 15 minutes with at least 30 minutes of follow-up). Since the complex compound is dextran-free, no anti-dextran antibodies are bound. Therefore, there is no increased risk of dextran-induced anaphylactic reactions.
Further reading:
- Klip IT, et al: Am Heart J 2013; 165: 575-582.
- Ponikowski P, et al: Eur J Heart Fail 2016; 18: 891-975.
- Okonko DO, et al: J Am Coll Cardio 2011; 58: 1241-1251.
- Jankowska EA, et al: Eur Heart J 2013; 34: 816-826.
- Hastka J, et al: Iron deficiency and iron deficiency anemia guidelines 2018.
- Hoes MF, et al: Eur J Heart Fail 2018; 20: 910-919.
- Cappellini MD, et al: Am J Hematol 2017; 92: 1068-1078.
- Lewis GD, et al: JAMA 2017; 317: 1958-1966.
- Laufs U, et al: DGK 2016; 1: 1-65.
- Anker SD, et al: Eur J Heart Fail 2018; 20: 125-133.
- Qunibi W, et al: Nephrol Dial Transplant 2011; 26: 1599-1607.
- Geisser P: Port J Nephrol Hyperert 2009; 23: 11-16.
- Neiser S, et al: Int J Mol Sci 2016; 17: 1185.
- Doehner W, et al: Dtsch Med Wochenschr 2017; 142: 752-757.
- Ronco C, et al: J Am Coll Cardiol 2008; 52 (19): 1527-1539.
- Kovesdy CP: Rev Endocr Metab Discord 2017; 18 (1): 41-47.
- Dunn JD, et al: Am J Manag Care 2015; 21 (15 Suppl): s307-s315.
- Bushinsky DA, et al: Kidney Int 2015; 88 (6): 1427-1433.
CARDIOVASC 2021; 20(1): 18-19