The spectrum of immunotherapies for multiple sclerosis is constantly expanding. This is because today’s options for long-term treatment can delay the progression of the disease in many patients – but not in all. However, attention should be paid in Switzerland primarily to the approval and other limitations, which may differ from those in the EMA area.
In Switzerland, approximately 15,000 people suffer from multiple sclerosis (MS) with increasing prevalence [1]. The chronic, as yet incurable neurodegenerative disease is associated with an unpredictable course. But treatment options have expanded. In recent years, immunotherapy has steadily gained in importance. More than a dozen compounds with different mechanisms of action, modes of administration, target populations, and benefit-risk profiles are now available for disease-modifying treatment of relapsing forms of MS. However, their approval in Switzerland may differ significantly from those in neighboring countries. Limitations due to the list of specialties also have an impact on the application. Also, progression-modifying therapy is now approved for patients with primary progressive MS. The goal of individualized therapy is moving ever closer. Aspects such as current life circumstances or perspective goals such as pregnancy or the professional situation can always be better taken into account when selecting the right treatment [2].
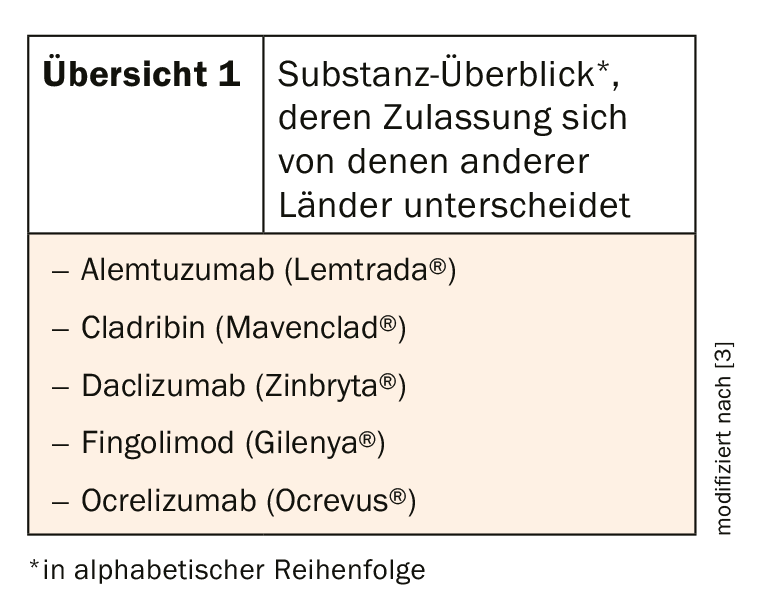
However, special regulatory conditions in Switzerland may lead to differences in the use of immunotherapeutics in clinical practice that are not reflected in European or national guidelines of other countries (overview 1) [3]. Different risk-benefit assessments, but also procedural aspects, can lead to these partially divergent approvals. This could also be due to the staggered submission to the regulatory authorities, which then access divergent data situations. This includes not only scientific results, but also other aspects such as data quality and product quality.
Possible side effects in view
The new substances are very effective, but in rare cases they can have potentially serious side effects. Therefore, regular neurological monitoring is essential. For example, cases of progressive multifocal leukoencephalopathy have been reported. Therefore, patients at high risk for developing PML should undergo cranial MRI after initiation of treatment. In addition, a lumbar puncture to determine JCV DNA prior to treatment initiation is recommended for this patient group [2].
Exceptional case: autologous hematopoietic stem cell transplantation
Autologous hematopoietic stem cell transplantation (aHSCT) has been shown to be effective for malignancies of the hematopoietic system (Box). Their use in MS therapy has also been researched for years. Again, it appears to be effective, but has a significant risk of side effects. Therefore, it is currently performed in Switzerland only in patients with a severe course under certain conditions [4]: the MS patients should be in the relapsing phase of the disease (in a primary/secondary chronic progressive course, inflammatory lesions in the CNS should still be present), several criteria must indicate an active/aggressive form of the course, the affected persons should preferably not be over 50 years of age and should not have severe impairments [5].
Effective treatment strategy
The NEDA status (“No Evidence of Disease Activity”) has become common to indicate the effectiveness of a treatment. This term describes the absence of clinical disease activity (relapses, disability increase) and MRI lesions. With highly effective treatment strategies such as immunotherapy, this can be achieved in up to 50% of patients over two years. However, by aHSCT, the fraction of patients with NEDA at two years is 70-92% [4]. However, cancer (<2%) and other autoimmune diseases (≤5%; especially inflammation of the thyroid gland with subsequent hypofunction) may occur as long-term side effects. In addition, the treatment may cause infertility in women, and very rarely in men.
Literature:
- Blozik E et al: Epidemiology and costs of multiple sclerosis in Switzerland: an analysis of health-care claims data, 2011-2015. Neuropsychiatr Dis Treat 2017; 13: 2737-2745.
- Chan A, Diem L. Indication and safety of course-modifying therapies for multiple sclerosis. Leading Opinions, Oct. 31, 2019. https://ch.universimed.com/fachthemen/1000001995 (last accessed 03/18/2020)
- Achtnichts L, Chan A, Czaplinski A, et al: Specifics of immunotherapy for multiple sclerosis in Switzerland. A structured report. SWISS MEDICAL FORUM – SWISS MEDICAL FORUM 2019; 19(41-42): 676-685.
- www.multiplesklerose.ch/PDF/de/News/2017/Stellungnahme_aHSCT_Wiss.Beirat_MS-Gesellschaft.pdf (last accessed 03/18/2020)
- www.multiplesklerose.ch/de/aktuelles/detail/stammzelltherapie-wird-von-krankenkasse-uebernommen/ (last accessed 3/18/2020).
InFo NEUROLOGY & PSYCHIATRY 2020; 18(2): 26-27.