Nephroprotection includes interventions that slow GFR decline over time, thereby delaying end-stage renal failure. The most recent intervention is the use of SGLT2 inhibitors in patients with diabetic nephropathy.
Chronic renal failure can be diagnosed in two ways:
- Evidence of impaired renal function with a calculated glomerular filtration rate (eGFR) of less than 60 ml/min that has persisted for at least three months.
- Evidence of chronic renal damage (albuminuria, hematuria, imaging evidence such as polycystic kidneys) persisting for at least three months – regardless of eGFR.
The diagnosis of chronic renal insufficiency can thus be made in practice with three simple examinations: Creatinine determination (calculate eGFR from this), urine status with sediment and proteinuria (spot urine) and renal sonography.
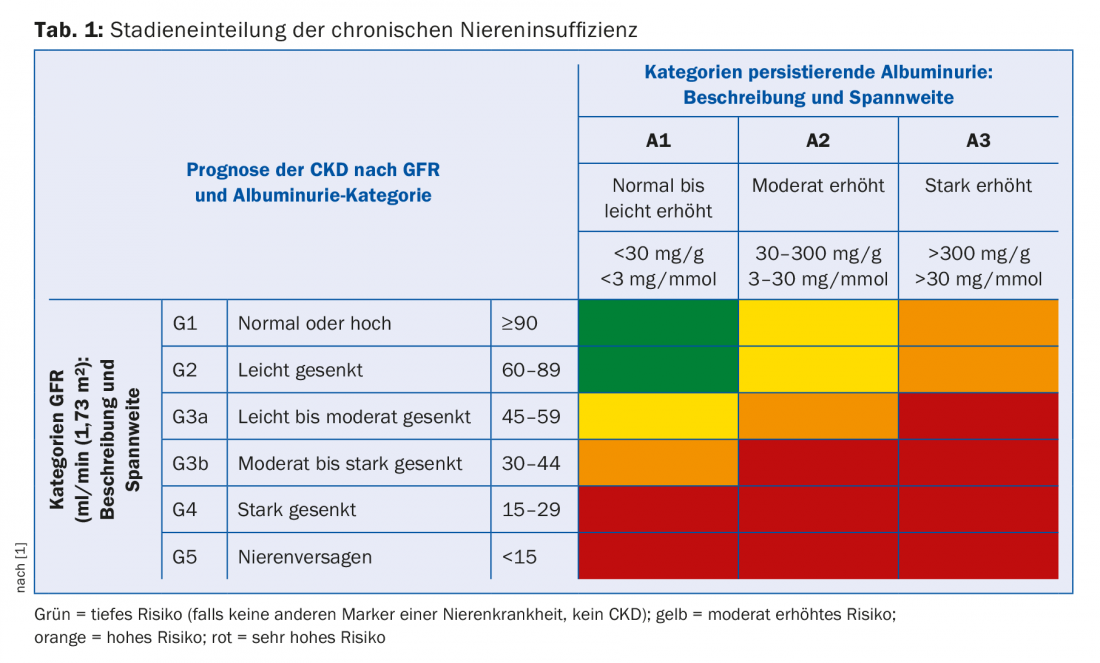
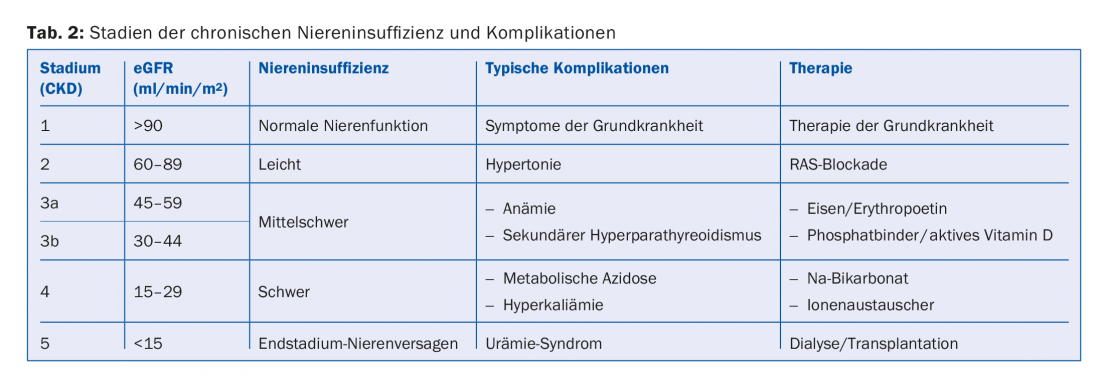
Depending on the extent of renal function impairment and albuminuria, chronic renal failure is staged. eGFR and albuminuria are independent risk factors for both cardiovascular mortality and the risk of developing end-stage renal failure [1]. The staging therefore indicates how often such patients should be monitored and how aggressively they should be treated with regard to cardiovascular risks (Tab. 1). Furthermore, the extent of renal function impairment indicates which secondary complications are to be expected (Tab. 2). These should be actively sought and, if necessary, treated.
Course
Chronic renal failure is progressive over the years, whether or not the underlying disease is still active. This phenomenon is explained by the so-called “Brenner hypothesis”, which states that with the loss of a larger number of nephrons, the remaining nephrons at least partially compensate for the loss of function and thus hyperfiltrate. This hyperfiltration, which persists for a long time, leads to damage of the still intact nephrons. Histologically, glomerular hypertrophy can be detected first, followed by increasing glomerulosclerosis, resulting in progressive loss of function and appearance of albuminuria.
The progression of chronic renal failure can be described individually per patient using the so-called Mitch curve [2]. If the eGFR or the value 1/creatinine is plotted against time, a linearly decreasing curve is obtained. This curve can be used to estimate the risk of developing end-stage renal failure for an individual patient (Fig. 1A). This facilitates discussion with the patient and allows timely preparations to be made for initiating a renal replacement procedure.
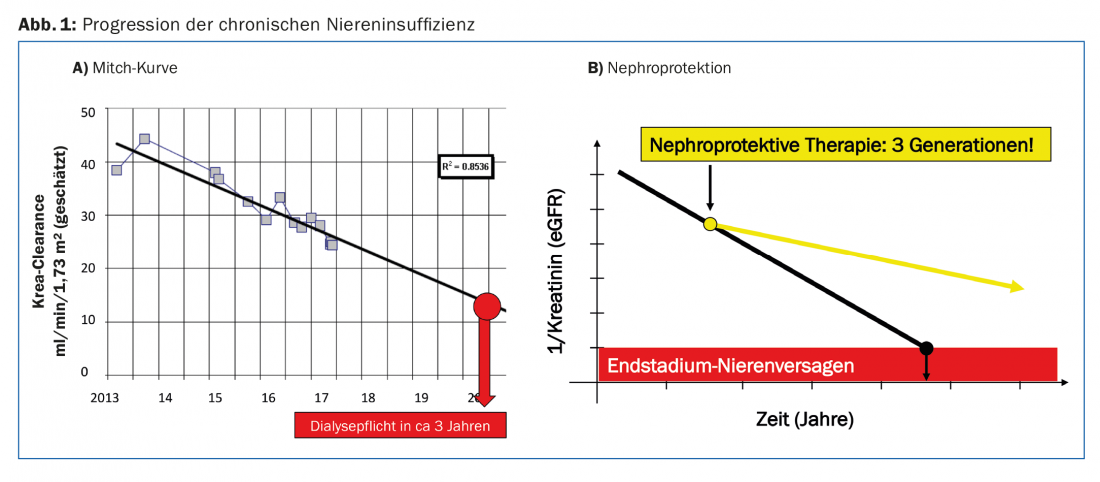
We as physicians would like to favorably influence the progression of chronic renal failure. By “nephroprotective measures” we mean all interventions that slow the decline in eGFR over time. To date, there has been no intervention to stop this waste completely. But if the drop in the Mitch curve can be flattened just a little, the patient may gain several years of dialysis-free time (Fig. 1B).
Nephroprotection
First generation – blockade of the renin-angiotensin system: The renin-angiotensin system (RAS) is crucially involved in the regulation of renal blood flow and thus the perfusion of the individual glomeruli. Angiotensin directly affects the tone of the vas efferens. More angiotensin leads to constriction of the vas efferens and thus to an increase in filtration pressure in the glomerular capillary bed. On the one hand, this allows autoregulation of glomerular filtration over a wide range of blood pressure variations. On the other hand, it also leads to the above-mentioned hyperfiltration in the context of the Brenner hypothesis. Blocking the RAS with ACE inhibitors, type 1 angiotensin receptor blockers (sartans), or renin inhibitors results in dilatation of the efferent vessel at the glomerulus. This reduces glomerular perfusion pressure and protects the glomerular capillary bed. At the same time, however, the glomerular filtration rate per nephron and overall decreases. Thus, initiation of RAS blockade in the renally insufficient patient always leads to a creatinine increase. Such an increase may be tolerated up to 25%, provided that it subsequently remains stable at this level. The creatinine increase is functional and evidence that glomerular perfusion pressure was indeed reduced. If the RAS blockade is stopped again, the creatinine drops again by the same value. If creatinine levels do not stabilize after the introduction of RAS blockade, it must be stopped again and a renal artery stenosis must be sought.
Several randomized trials in the 1990s demonstrated that RAS blockade can slow the progression of chronic renal failure, in both diabetic and nondiabetic nephropathy [3]. Thus, RAS blockade is now the basis of any nephroprotective intervention and should be made more aggressive the higher the proteinuria. Individual substances should be dosed to the maximum, and double RAS blockade should be strictly avoided [4].
Second generation – correction of metabolic acidosis: the kidney is the main organ for the excretion of fixed acids, and these accumulate with increasing limitation of renal function. Chronic renal failure is therefore usually associated with the occurrence of metabolic acidosis, especially in the advanced stages from CKD G3b.
Metabolic acidosis has several adverse effects. It impairs bone health and promotes the occurrence of hyperkalemia, which in turn increases the risk of cardiac arrhythmias. However, whether metabolic acidosis influences the progression of chronic renal failure itself was unclear for a long time. Then, in the 2000s, several randomized trials have been published where the influence of treatment of metabolic acidosis with sodium bicarbonate on the progression of chronic renal failure was investigated. To the surprise of many nephrologists, it has been shown that in various stages of chronic renal failure (CKD G2, G3, and G4), progression can be slowed and the onset of end-stage renal failure delayed with this simple measure [5]. Thus, it is now a general recommendation that metabolic acidosis with a serum bicarbonate <20 mmol/l should be treated with sodium bicarbonate. This also improves hyperkalemia, which in turn allows the most efficient RAS blockade to be maintained.
Limitations of this intervention often include tolerability (sodium bicarbonate causes flatulence), high tablet count (to achieve a serum bicarbonate >20 mmol/l sometimes requires six to eight tablets per day), and sodium loading, which can lead to increased edema and/or blood pressure elevation.
Third generation – blockade of the sodium/glucose cotransporter SGLT2 in the proximal tubule: In recent years, a new group of oral antidiabetic drugs has been tested in several randomized trials: These are inhibitors of the sodium/glucose cotransporter in the proximal tubule (SGLT2, “sodium-glucose transporter 2”). These drugs induce glucosuria. This results in a better HbA1c and a negative calorie balance leading to a net weight loss. At the same time, however, blockade of SGLT2 also leads to natriuresis. These agents are thus a new class of diuretics that, unlike loop diuretics and thiazides, act in the proximal tubule rather than the distal nephron.
SGLT2 inhibitors have been tested primarily in patients with type 2 diabetes and an elevated cardiovascular risk profile (often in secondary prevention). A highly significant benefit on all-cause mortality (empagliflozin) and cardiovascular mortality (empagliflozin, canagliflozin) was shown. Due to the diuretic effect, rehospitalizations due to decompensated heart failure were also significantly reduced [6,7].
Follow-up studies have now also shown a highly significant positive effect on hard renal endpoints (new onset of macroalbuminuria, doubling of serum creatinine, occurrence of end-stage renal failure). The effect is of the magnitude of RAS blockade in a patient group that is for the most part already treated with RAS blockers [8]! The mechanism of nephroprotection has not yet been elucidated in detail. However, an effect via the macula densa with dilatation of the vas afferens is postulated. The same functional creatinine increase at baseline with subsequent stabilization of renal function as with RAS blockade has been demonstrated [9].
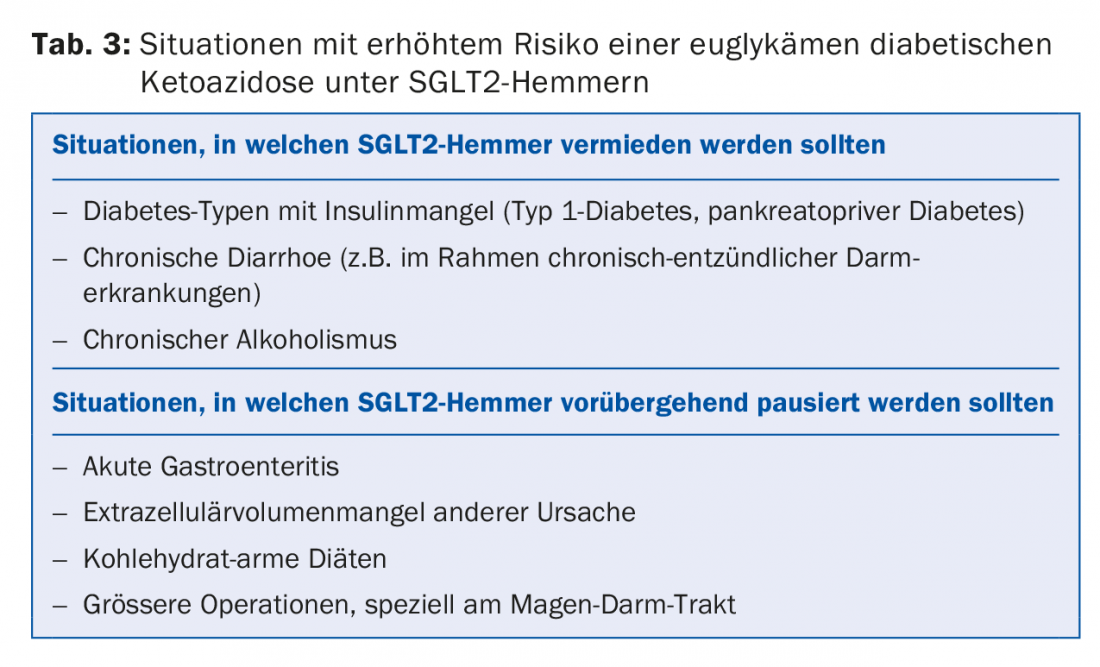
Two clinical side effects should be noted with this substance class: Frequent but harmless are increased urogenital infections, which can be easily treated. A rare but serious complication is euglycemic diabetic ketoacidosis, which can occur with insulin deficiency and long periods of fasting. Therefore, in the clinical situations listed in Table 3, SGLT2 inhibitors should not be used or should be used with great caution or temporarily paused.
Conclusion
After RAS blockade and metabolic acidosis therapy, a third nephroprotective measure has now been available to us for two years: the use of SGLT2 inhibitors. At the moment, this is only approved for patients with diabetic nephropathy (tab. 4) . Since the mechanism of nephroprotection most likely has nothing to do with the influence on glucose metabolism but with the diuretic effect of these substances, it can be assumed that they would also be effective in non-diabetic nephropathy. The corresponding studies are currently underway.

Take-Home Messages
- Chronic renal failure has a progressive course that can be described by the Mitch curve.
- By nephroprotective measures, we mean interventions that can slow GFR decline over time and thus delay end-stage renal failure.
- The basis of any nephroprotection is the use of inhibitors of the renin-angiotensin system (ACE inhibitors, AT1 receptor blockers, renin inhibitors). These should be dosed according to compatibility, but not combined.
- Treatment of metabolic acidosis with sodium bicarbonate is nephroprotective and should be started at a serum bicarbonate <20 mmol/l.
- The most recent intervention is the use of SGLT2 inhibitors in patients with diabetic nephropathy. They have a diuretic effect on the proximal tubule and are cardio- and nephroprotective.
Literature:
- Stevens PE, Levin A: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825-830.
- Mitch WE, et al: A simple method of estimating progression of chronic renal failure. Lancet 1976; 2: 1326-1328.
- Lewis EJ, et al: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329: 1456-1462.
- Luft FC: Perspective on combination RAS blocking therapy: off-TARGET, dis-CORD, MAP-to-nowhere, low ALTITUDE, and NEPHRON-D. Am J Nephrol 2014; 39: 46-49.
- de Brito-Ashurst I, et al: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20: 2075-2084.
- Zinman B, et al: Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117-2128.
- Neal B, et al: Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377: 644-657.
- Wanner C, et al: Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016; 375: 323-334.
- Anders HJ, et al: Nephron Protection in Diabetic Kidney Disease. N Engl J Med 2016; 375: 2096-2098.
HAUSARZT PRAXIS 2018; 13(4): 22-25
CARDIOVASC 2019; 18(5): 6-9