The biologic Dupilumab has proven itself under “real-life” conditions and has already received an extension of approval in the EU for the age group 12 years and older. At the SGDV annual meeting, experts provided information on the current state of research.
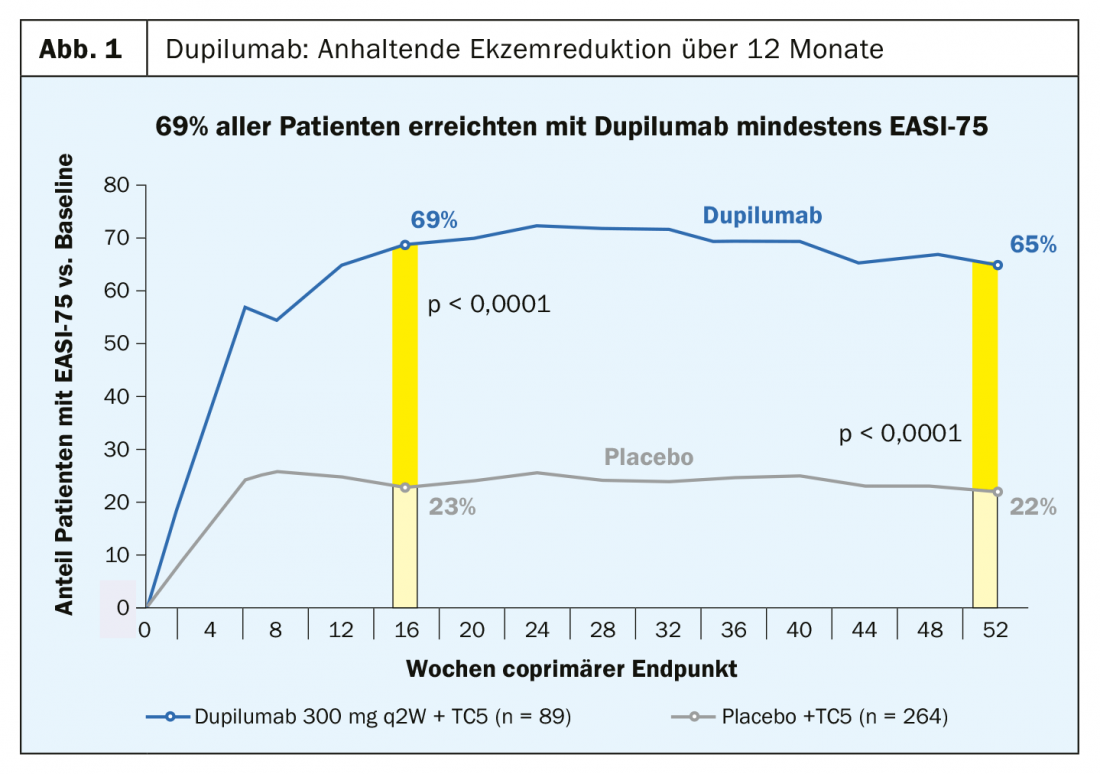
The recombinant human IgG4 monoclonal antibody dupilumab, approved in Switzerland for moderate to severe atopic dermatitis in adults since this year, inhibits signal transduction of IL4 and IL13, two key cytokines in the inflammatory process. In the phase III LIBERTY AD CHRONOS trial, 69% of subjects treated with dupilumab achieved at least EASI-75, significantly more than in the placebo condition (p<0.0001) [1,2] (Fig. 1).
Positive “real life” interim balance sheet
Registry data from Germany of adult patients with atopic dermatitis who are suitable for system therapy show a positive picture for dupilumab under “real-life” conditions [1]. Longitudinal studies are currently underway to monitor newly initiated systemic therapies. An interim evaluation after three months with more than 100 patients showed that an improvement in EASI75 was measurable in 57% of patients, which was a positive result, the speaker said [1]. In the EU, where Dupixent® has already been approved for adults with moderate to severe atopic dermatitis since 2017, a marketing authorization extension for adolescents aged 12 years and older was recently granted [3].
The drug is very easy to handle in practice: the recommended dosage of Dupixent® in adults is an initial dose of 600 mg as a subcutaneous injection (two injections of 300 mg each), followed by a dose of 300 mg as a subcutaneous injection every two weeks [4]. It is explicitly stated that topical therapy should not be suspended when starting systemic therapy with dupilumab. In cases of patients suffering from asthma in addition to atopic dermatitis treated with oral corticosteroids, the steroid dose can be reduced [4], the speaker explained. However, it is important to keep this in mind when discontinuing dupilumab therapy and to advise the patient to readjust the steroid dose [1]. Regarding treatment of possible side effect of atypical dupilumab-induced conjunctivitis, which is a different type of ocular inflammation than allergic conjunctivitis, Prof. Werfel and colleagues recommend the following [5]: 1. eyelid margin care and tear substitute, 2. prescribe eye drops (fluorometholone or hydrocortisone), 3. if symptoms do not heal: Refer to ophthalmologist who can prescribe eye drops containing ciclosporin. In the technical information resp. the package leaflet also lists oral herpes as a common side effect. Prof. Werfel is critical of this assessment because this side effect was also common in placebo groups. With regard to eczema herpeticatum, the risk is not increased but is reduced by about 60%, emphasizes Prof. Werfel [6].
Guideline recommendation as first-line therapeutic emotion
Dupilumab and ciclosporin are recommended in current guidelines as first-line systemic therapeutics for chronic moderate to severe atopic dermatitis in adults [7–9]. Methotrexate, azathioprine, and mycophenolate mofetil can be used as off-label preparations. Systemic glucocorticosteroids are not indicated as long-term therapy (>3 weeks); these should only be used in the short term for acute episodes, the speaker explained [1]. Specific immunotherapy plays a minor role in atopic dermatitis according to current knowledge. The authors of a Cochrane meta-analysis concluded that there is limited evidence for the efficacy of spcific immunotherapy (SIT) in atopic dermatitis. However, it could be clearly proven that SIT in the indication of pollen sensitization and atopic dermatitis does not harm, i.e. it does not lead to a worsening of the atopic symptoms.
Translational research has more arrows in its quiver
Currently, several studies are underway to investigate the efficacy and safety of additional immunomodulatory agents in atopic dermatitis. According to Prof. Werfel, some of these have a good chance of achieving market approval in the coming years:
anti-IL13 antibodies: Representatives of this substance class act more specifically than dupilumab and attack only one of the two cytokines. Tralokinumab has shown good efficacy in studies to date; nearly 50% achieved EASI75 at 12 weeks at the 150 mg dose [1].
Janus kinase 1 and 2 inhibitors: According to current data, there is a very rapid onset of action, with very good effects measurable after just four weeks [1]. Baricitinib is already currently available in Germany in rheumatology, said Prof. Werfel. He has high hopes that approval will be granted for the indication of atopic dermatitis.
anti-IL31 antibody and anti-TSLP: IL31 and TSLP are cytokines that both act as mediators of inflammation and are involved in itch [1]. Studies on efficacy in atopic dermatitis are not yet very advanced, he said.
Histamine-4 receptor antagonists: Like the two candidate molecules IL31 and TSLP, histamine-4 has a dual function in the pathomechanism of atopic dermatitis (inflammatory mediator, itch mediator). Currently, a large phase IIb trial is underway to investigate efficacy [1]. According to current knowledge, histamine-4 receptor blockers are more effective in atopic dermatitis than histamine-1 receptor and histamine-2 receptor antagonists.
Source: SGDV, Basel
Literature:
- Werfel T: Atopic dermatitis – newest therapeutic approaches for practice, Key Lecture 4, slide presentation, Prof. Dr. med. Thomas Werfel, Hannover Medical School, SGDV Annual Meeting, Basel 20.09.2019.
- Blauvelt A, et al: Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017 Jun 10; 389(10086): 2287-2303. doi: 10.1016/S0140-6736(17)31191-1. epub 2017 May 4.
- EMA: Dupixent®, www.ema.europa.eu/en/medicines/human/summaries-opinion/dupixent-0
- Dupixent® 300 mg: Expert information, https://compendium.ch
- Wohlrab J, Wollenberg A, Reimann H, et al: Interdisciplinary recommendation for action in dupilumab-associated ocular inflammatory disease. Der Hautarzt 2019; 70(1): 64-67.
- Fleming P: Risk of infection in patients with atopic dermatitis treated with dupilumab: A meta-analysis of randomized controlled trials. JAAD 2018; 78(1): 62-69.e1, https://doi.org/10.1016/j.jaad.2017.09.052
- AMWF: Guideline “Neurodermatitis”, www.awmf.org/leitlinien/detail/ll/013-027.html
- Werfel T. et al: S2k-Leitlinie Neurodermitis [atopic eczema; atopic dermatitis] Kurzversion, JDDG 2016, https://onlinelibrary.wiley.com/doi/abs/10.1111/ddg.140_12871
- European Dermatology Forum: EDF-Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis) Part I, www.isplad.org/wp-content/uploads/2018/03/linee_guida_eczema_atopico.pdf
DERMATOLOGIE PRAXIS 2019; 29(5): 30-31 (published 10/10/19, ahead of print).