Regular physical training can induce structural, electrical and functional adaptations of the heart. Exercise can be a trigger for exercise-induced tachyarrhythmias or exercise-induced sudden cardiac death (SCD) in the presence of underlying cardiac disease. In order to identify athletes with underlying cardiac disease and increased risk for SCD at an early stage, “preparticipation screening” is recommended in Europe. Preparticipation screening includes a resting ECG in addition to a specific medical history and cardiac auscultation; it is recommended beginning at age 14 and should be repeated at least every two years until competitive activity is discontinued. In athletes older than 35 years, coronary artery disease dominates as the most common cause of SCD; consequently, the cardiovascular risk profile should be taken into account in athletes in this age group.
Repeated, prolonged, and high-intensity exercise can cause physiological adaptations of a structural, electrical, and functional nature in the heart of competitive athletes, which are subsumed under the term “athlete’s heart.” These changes are sometimes difficult to distinguish from heart disease, which is associated with an increased risk of sudden cardiac death.
Sports-associated sudden cardiac death
Sports-associated sudden cardiac death (SCD) is defined as “unexpected death of cardiac origin occurring during or within one hour of exercise.” The incidence is very low in athletes <35 years and is 0.5-3/100 000/year depending on the collective studied. Accidental deaths in athletes may be up to three times more common. Some lack of understanding of SCD events, which are perceived with great public concern, results from the fact that athletes are considered the healthiest segment of the population and physical activity is commonly associated with a reduction in cardiovascular mortality. Men are more than twice as likely to die while playing sports compared to women. Sports-associated SCDs occur four times more frequently in black athletes than in white athletes.
Sport is not the cause, but only a trigger for load-dependent tachyarrhythmias, which usually occur on the background of an existing heart disease. Because athletes are often asymptomatic at rest, sudden cardiac death may be the initial manifestation of the disease. In athletes younger than 35 years, congenital cardiovascular diseases such as hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), coronary anomalies, and ion channel diseases (e.g., long-QT syndrome) predominate. In addition, myocarditis is a frequently underestimated cause. Coronary artery disease dominates in athletes over 35 years of age. Intense “stop-and-go” ball sports such as football or basketball, as well as cycling, triathlon, and strength sports with high intensity peaks (e.g., weightlifting) are associated with an increased risk of SCD.
Competitive athletes have significantly higher risk, but the definition of competitive sports is not uniform. Competitive athletes engage in regular training and take part in competitions, i.e. organized sporting events (team or individual) from the point of view of performance and sporting success. Characteristic of competitive athletes is their willingness to push themselves physically to the limit with the goal of improving performance.
Preparticipation Screening
In order to identify athletes with underlying cardiac disease and thus increased risk for SCD at an early stage, a preparticipation screening is recommended in Europe, which includes a resting ECG in addition to a specific medical history and cardiac auscultation. This strategy has been adopted by the Swiss Society of Sports Medicine (SGSM) in its recommendations for the prevention of SCD in young athletes. In 1998, the first official recommendations for the prevention of SCD in young athletes were published. Currently, the SGSM recommends that all competitive athletes, and in particular squad athletes over the age of 14, undergo a sports medical examination, including a medical check-up. Perform resting ECG every one to two years until retirement from competition.
Critically, the benefit of these screening recommendations is controversial, as a benefit has only been shown in a small observational study from northern Italy. The question of cost efficiency is also raised again and again in this context.
The standardized criteria for ECG assessment in athletes have been continuously adapted in recent years due to the very high rate of false-positive findings with subsequent (often expensive) additional investigations. Based on the 2010 European Society of Cardiology (ESC) recommended criteria, the rate of positive findings was 9.6%, of which only 10.3% of those screened effectively had a cardiac abnormality.
Exercise-associated ECG changes
In the current “Seattle Criteria” of 2013, this rate is still 4.2% as a result of various adjustments. The “Seattle Criteria” differentiate between ECG changes that are typical for athletes or associated with training and abnormal ECG changes that are to be considered pathological.
The former group (Table 1) subsumes virtually exclusively changes attributable to increased vagotonus or structural adaptations of the heart to exercise.

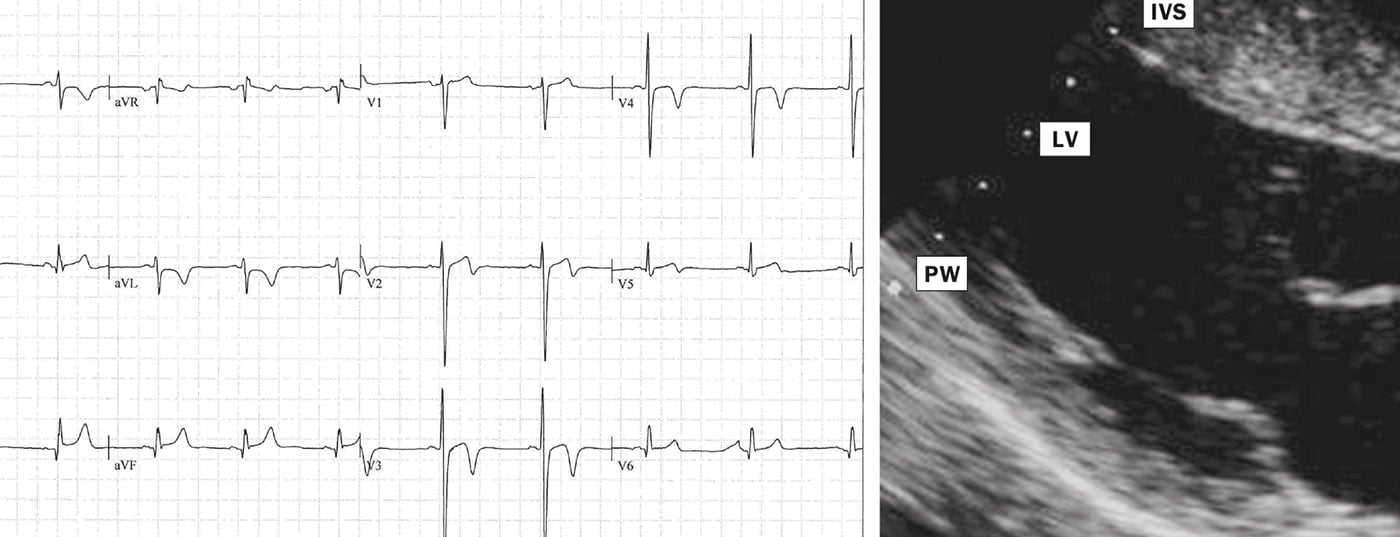
These include sinus bradycardia >30 bpm, sinus arrhythmia, ectopic atrial rhythms, junctional replacement rhythm, AV block I. Grades (PR interval >200 ms), AV block II. Grade Mobitz Type I (Wenckebach), incomplete right bundle branch block, and various patterns of early repolarization. Athletes of black skin color may exhibit an ethnic variant of early repolarization (Fig. 1).
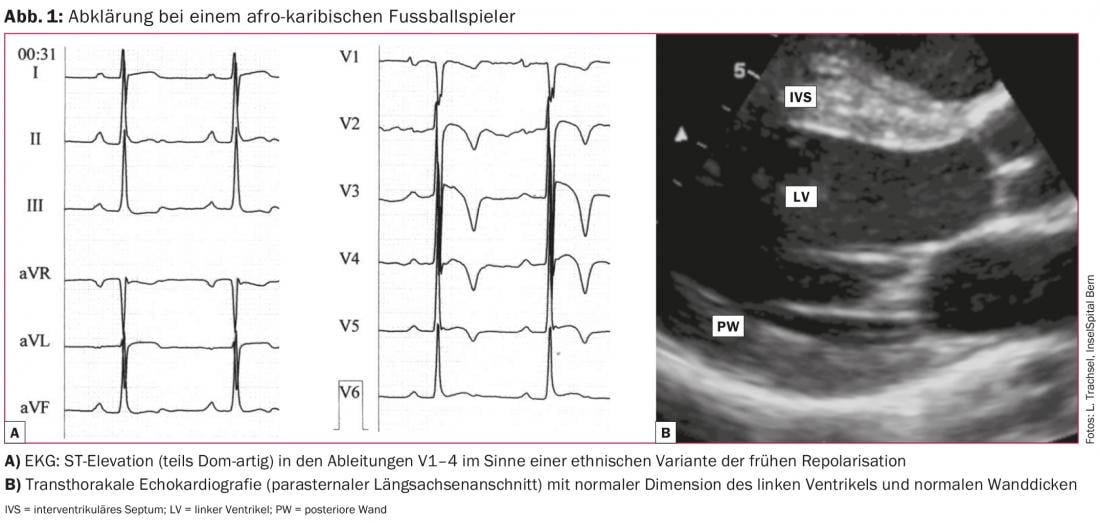
In more than half of the athletes, these ECG changes persist after completion of the athletic career, with no negative outcome at an observation interval of >20 years. In summary, all of these exercise-associated findings do not require further clarification.
Abnormal ECG changes
Abnormal changes in resting ECG such as T-negativations (except leads II, aVR, and V1), left anterior fascicular block, complete bundle branch block images, ventricular preexcitation, or prolonged corrected QT time are much less common (Tab. 2). They are not exercise-associated and potentially reflect underlying structural or electrical heart disease with increased risk of exercise-associated sudden cardiac death. Consequently, they require extensive cardiologic evaluation.

In the general population, hypertrophic cardiomyopathy (HCM) is the most common cardiomyopathy with a prevalence of 0.2%. It is significantly lower in athletes (0.07-0.08%), possibly because of the generally somewhat reduced performance of patients with HCM. In more than 90% of cases, HCM presents with an abnormal resting ECG, and T negativities in the chest and/or extremity leads are crucial for diagnosis (Fig. 2).
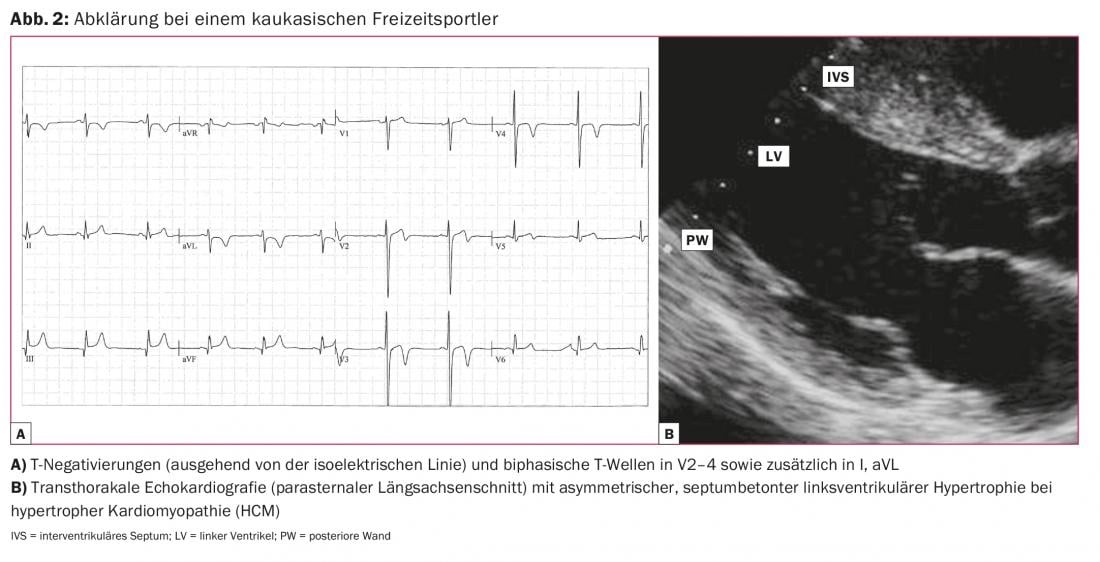
T-negativities may also occur on the ECG in arrhythmogenic right ventricular cardiomyopathy (ARVC), dilated cardiomyopathy (DCM), and after myocarditis. T negatives should always be evaluated by cardiac imaging, primarily using transthoracic echocardiography. In addition, cardiac magnetic resonance imaging (MRI) provides important additional information for diagnosis or exclusion of the mentioned diseases because of better visualization of myocardial segments, especially the right ventricle, and evidence of fibrosis and inflammation. If coronary anomaly is suspected, MR angiography can visualize the ostia and course of the proximal coronary vessels. The often difficult distinction between physiological adaptation in the context of (endurance) sport and pathological change in the sense of a disease is facilitated by these examination modalities, but is still a great challenge at times.
For the electrical abnormalities such as ventricular preexcitation, congenital long-QT, and Brugada syndrome, the diagnosis is made with the 12-lead ECG. In Wolff-Parkinson-White(WPW) syndrome, electrophysiologic testing with ablation of the accessory pathway may allow the athlete to continue competitive activity. Competitive sports are not recommended in cardiomyopathies and long QT syndrome.
In athletes in the age group over 35 years, coronary heart disease in particular must be considered. In this case, depending on the activity level, a cardiological evaluation is recommended, which, in addition to a medical history and physical examination, also includes an assessment of the cardiovascular risk (e.g., using the AGLA score). In the case of abnormal findings, ergometry should also be performed to search for relevant coronary stenosis. However, it must be critically noted that hemodynamically irrelevant but rupture-prone coronary artery plaques may be present despite clinically and electrically negative ergometry.
Literature:
- Drezner JA, et al: Electrocardiographic interpretation in athletes: the “Seattle criteria”. Br J Sports Med 2013; 47(3): 122-124.
Further reading:
- Maron BJ, Pelliccia A: The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation 2006; 114(15): 1633-1644.
- Corrado D, et al: Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Eur Heart J 2005; 26(5): 516-524.
- Marti B, Hintermann M, Lerch R: Sudden cardiac death in sports: useful screening and prevention measures. Swiss Journal of Sports Medicine and Sports Traumatology 1998; 46(2): 83-85.
- Steinvil A, et al: Mandatory electrocardiographic screening of athletes to reduce their risk for sudden death proven fact or wishful thinking? J Am Coll Cardiol 2011; 57(11): 1291-1296.
- Weiner RB, et al: Performance of the 2010 European Society of Cardiology criteria for ECG interpretation in athletes. Heart 2011; 97(19): 1573-1577.
- Borjesson M, et al: Cardiovascular evaluation of middle-aged/senior individuals engaged in leisure-time sport activities. J of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology 2011; 18: 446-458.
CARDIOVASC 2015; 14(2): 10-13