In the treatment of severe traumatic brain injury, there is much literature and little evidence. Strict adherence to a treatment protocol leads to better outcome; moreover, treatment should be provided in specialized centers. Causes and triggers for secondary damage must be aggressively sought and treated. Normocapnia, normoxemia, normotension, normoglycemia, normothermia, and normal ICP resp. CPP are the target. The treatment of the whole organism is carried out according to the “good clinical practice in intensive care medicine”. A reliable serious prognosis can only be made in the long term. Rash decisions based on image morphological data are not advised.
Intensive care treatment after severe traumatic brain injury (SHT) presents a challenge to the entire treatment team. Involved are intensive care physicians, intensive care nurses, neurosurgeons, neurologists and other specialists from the fields of speech therapy, occupational therapy and physiotherapy. Interdisciplinary treatment in particular requires close coordination and management of specialists. Therefore, the treatment of severe SHT in adults in Switzerland may only be carried out in centers with the necessary infrastructure and expertise. In most ICUs, patients are treated according to a strict treatment protocol [1–5]. Although evidence for the superiority of one treatment protocol or another is lacking [6], studies show that following and adhering to a protocol generally benefits the patient [7].
In Switzerland, approximately 715 persons per year suffer a severe SHT [8]. The severity of injury is most often determined using the Glasgow Coma Scale (GCS) or the Abbreviated Injury Score (AIS). Severe SHT is defined as GCS <9 or AIS “head” four or five and has great socioeconomic importance.
Intensive care is an important, but not the sole critical, part of the treatment chain in the care of severe SHT. The principles of intensive care are also applied in upstream settings, beginning with prehospital emergency care, continuing through the clinical emergency care and surgical phases, and ending with entry into the intensive care unit.
The primary damage, which is caused by a direct or indirect force impact on the head and brain lasting a very short time (<0.2 msec), is called primary damage. Depending on the cause of the accident (high-speed accident), the intensity and direction of the force (acceleration or rotational forces), this is manifested in laceration-crush wounds, cranial dome fractures (Fig. 1 ) , extraaxial (Fig. 2) and intracerebral hemorrhages, cerebral tissue contusions, perifocal or generalized cerebral edema, and shearing injuries of the neurons (“shearing injuries”). This structural damage can be detected by cCT or cMRI and cannot be reversed by any therapy. After the initial emergency CT angio in the shock room (depending on the cause of the accident, the course of events, etc., even a polytrauma CT must be performed), an interdisciplinary decision must be made as to whether surgical intervention or a conservative approach is necessary or not. makes sense.

Avoid secondary damage
The basic principle in the treatment of SHT is the prevention of so-called secondary damage, i.e. damage that occurs after the initial insult. The main focus is on sufficient oxygen supply to the brain. Therefore, on the one hand, everything is done to enable an adequate supply of oxygen to the brain, and on the other hand, the brain’s oxygen consumption is minimized. This includes aggressive volume and catecholamine therapy, controlled ventilation with narrow target values regarding paO2 and paCO2, and deep analgesia (benzodiazepines, propofol, and opioids) of the patient.
Cerebral perfusion pressure (CPP) is still used as a marker of adequate oxygenation in the absence of clinically feasible techniques. This is the pressure difference between mean arterial pressure (MAP) and intracerebral pressure (ICP) (CPP = MAP-ICP). This requires the invasive measurement of ICP [9]. Most commonly, an intracranial pressure probe is inserted into the cerebral parenchyma or ventricular system. This allows the intracranial pressure to be measured continuously. A CPP >60 mmHg is currently considered sufficient in most guidelines. Other investigative techniques that may be used during the course of treatment, depending on the problem, include electrophysiology (EEG, SSEP), transcranial Doppler studies, and perfusion measurements.
Multimodal cerebral monitoring with local measurement of brain tissue metabolism, such as cerebral microdialysis (lactate, pyruvate, glutamate, and glycerol), tissue O2 measurements (ptO2), and brain temperature, has not yet been able to establish itself as a standard. Currently, there is a lack of clinical studies that have been able to show a benefit for the patient when using multimodal monitoring, because mostly so-called surrogate markers are analyzed.
Because the brain is enclosed by the bony skull and dura mater, any intracerebral increase in volume (cerebral edema, hemorrhage, CSF accumulation, vascular dilatation venous or arterial), after exhaustion of compensatory mechanisms, leads to an increase in ICP and thereby compromises intracerebral blood flow (Monro-Kellie doctrine). Therefore, control of ICP in addition to CPP is another key therapeutic target.
ICP increases may be intracerebral (volume increase intracerebrally) or extracerebral in origin [10]. Extracerebral causes of ICP increase include hypoventilation/hypercarbia (cerebral vascular dilatation with paCO2 increase), hyponatremia (enhancement of cerebral edema), blood pressure drops, hypoxemia, fever due to extracerebral infections, and coagulation disorders. Hyper- or hypoglycemia causes an energetic deficit.
Any coagulopathy must generally be aggressively treated because coagulopathy causes rebleeding in traumatized brain tissue. The immobility of patients with severe SHT increases the thromboembolic risk, which is not treated with medication but only with physical measures such as anti-thromboembolic stockings (ATS) and sequential inflatable stockings (“sequential stocking devices”, SCD) in the first days.
CNS damage leads to neuroinflammation with formation of vasogenic or cytotoxic brain edema with concomitant ICP increase, necrosis, and/or apoptosis of neurons. Administration of high-dose steroids fails to suppress this neuroinflammation and even leads to worse neurological outcome [11,12]. Discontinuation of steroid administration is one of the very few recommendations in the treatment of severe SHT with grade I evidence.
If initial conservative or postneurosurgical therapy does not stabilize the ICP, emergency imaging must be performed to rule out postoperative hemorrhage, cerebral edema, or CSF congestion (Fig. 3) . Together with the neurosurgeons, a surgical option is discussed such as hematoma evacuation, hemicraniectomy [13] and/or CSF drainage. If no surgical option is possible, further escalation is performed in the staged regimen (deeper analgesia, muscle relaxation, osmotherapy with mannitol or hypertonic saline, therapeutic hypothermia, and barbiturate coma) [14].
After stabilization, which often takes several days, a recovery attempt can be performed under strict control of the ICP. This awakening phase is characterized by a massive stress reaction of the body, which must be suppressed accordingly with medication.
Treat whole organism
Another basic principle is to establish homeostasis and treat the whole organism, not just the SHT. Especially in the case of multiple injuries, organ-specific treatment principles may compete with each other, such as in the case of severe lung injury with protective ventilation strategy in combination with severe SHT (no hypoventilation because of vasodilation and ICP increase).
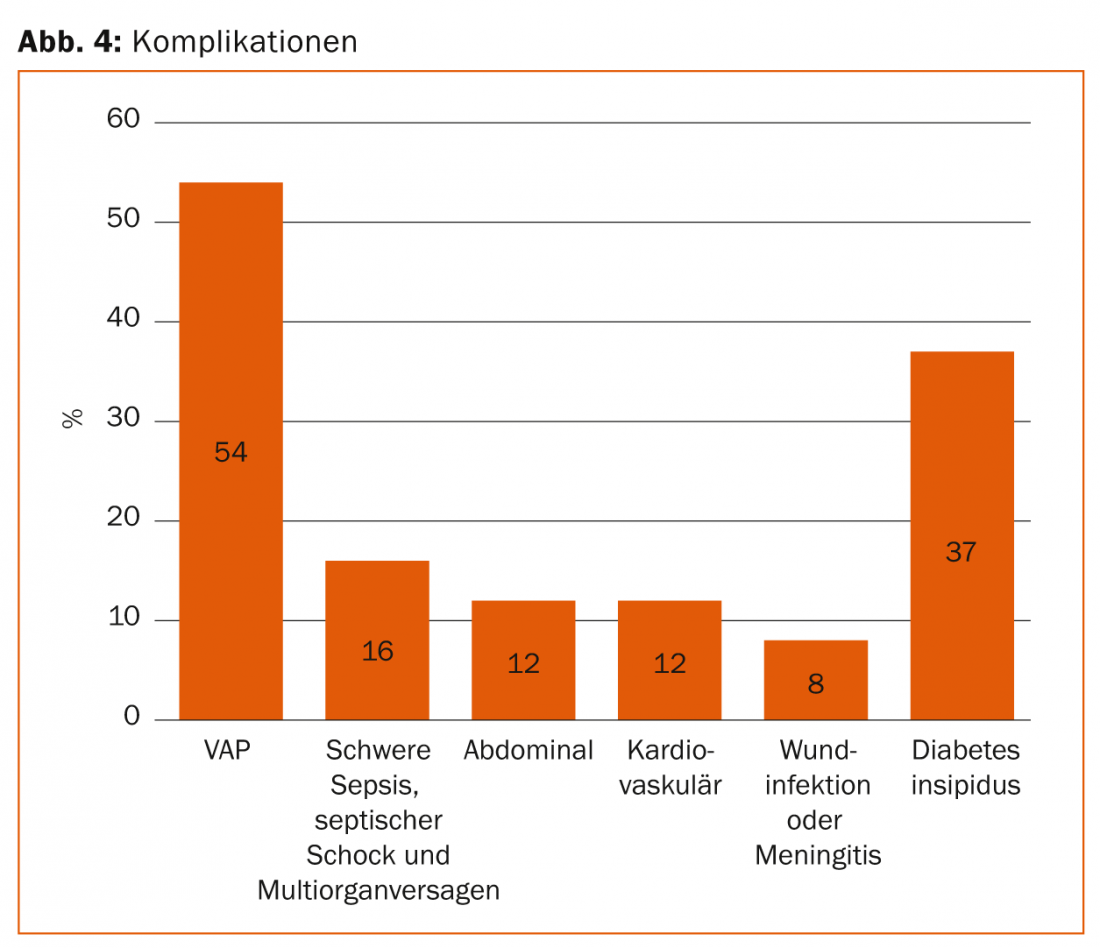
Regular long-term intensive care leads to complications such as ventilator-associated pneumonia (VAP), intestinal paralysis with problematic nutritional buildup, translocation of intestinal bacteria with subsequent sepsis, severe catabolism (muscle breakdown), and multiorgan failure ( Fig. 4).
Neurorehabilitation and prognosis
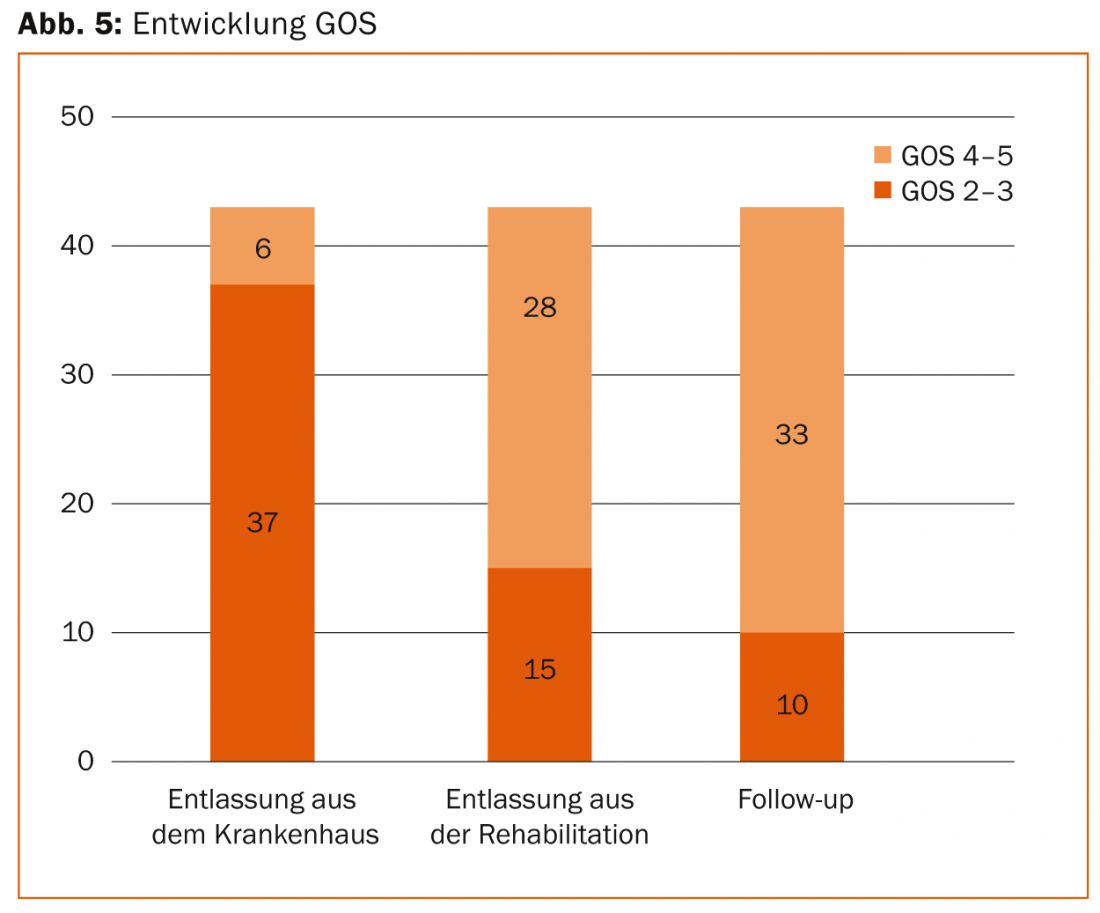
Every patient with a severe SHT requires several weeks to months of neurorehabilitation after the intensive care phase. Early neurorehabilitation, which is initiated during the recovery phase in the intensive care unit and subsequently continued in specialized clinics, can bring about a significant improvement in neurology in many cases. For example, our own retrospective data showed that three quarters of patients with a severe SHT left the ICU with severe neurological deficits (GOS 2-3) to neurorehabilitation, only to return independently to daily life months later from neurorehabilitation in three quarters of cases in good neurological condition (GOS 4-5) (Fig. 5).
Therefore, a reliable neurological prognosis can only be established after several weeks, months or only after one year if the critical initial unstable phase is survived. This is because most patients with severe SHT die in the first few days because of uncontrollable ICP, which leads to cerebral perfusion arrest and thus brain death. In such situations, the question of organ donation arises, which must be discussed with the relatives – in accordance with the presumed will of the patient – because young patients rarely have a living will or an organ donor card. Thus, the intensive care team is very challenged in choosing the intensity of treatment. Elderly and aged persons most frequently suffer a severe SHT, and due to the age-related limited organ reserves and the lack of rehabilitation potential, the question of treatment intensity arises quite explicitly. Especially with this injury pattern, the maxim “survival at all costs” is the wrong advisor. The neurological sequelae are often so severe that the intensive medical therapy goal must be changed in accordance with the presumed will of the patient, leading to palliative therapy.
In most cases, patients after severe SHT have cognitive, motor, sensory, psychological and thereby social handicaps in varying degrees, which must be observed and taken into account by those providing follow-up treatment. Each patient must therefore be followed up in a completely individual and patient-centered way.
Useful links:
www.braintrauma.org
www.snacc.org
www.dgnc.de
www.pebita.ch
www.swissneurosurgery.ch
www.swissneuro.ch
www.awmf-online.de
Literature:
- The Brain Trauma Foundation: The American Association of neurological Surgeons; Congress of Neurological Surgeons: Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007; 24(Suppl 1): S1-S106.
- Maas AI, et al: EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Vienna) 1997; 139(4): 286-294.
- Grände PO: The “Lund concept” for the treatment of severe head trauma – physiological principles and clinical application. Intensive Care Med 2006; 32(10): 1475-1484.
- Welling KL, Eskesen V, Romner B, The Danish Neurotrauma Committee: Neurointensive care of severe traumatic brain injury. Ugeskr Laeger 2010; 172: 2091-2094.
- Menon DK: Cerebral protection in severe brain injury: physiological determinants of outcome and their optimisation. Br Med Bull 1999; 55(1): 226-258.
- Sundstrøm T, et al: Management of severe traumatic brain injury. Evidence, tricks, and pitfalls. Springer 2012. ISBN 978-3-642-28125-9.
- Gerber LM, et al: Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg 2013; 119: 1583-1590.
- Walder B, et al: Severe traumatic brain injury in an high-income country: An epidemiology study. Journal of Neurotrauma 2013; 30: 1934-1942.
- Chesnut RM, et al: A trial of intracranial-pressure monitoring in traumatic brain injury. New England Journal of Medicine 2012; 367(26): 2471-2481.
- Maas AI, Stocchetti N, Bullock R: Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008; 7(8): 728-741.
- CRASH trial collaborators: Effects of intravenous corticosteroids on death within 14 days in 1008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet 2004; 364: 1321-1328.
- CRASH trial collaborators: Final results of MRC CRASH: a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet 2005; 365: 1957-1959.
- Cooper DJ, et al. for the DECRA Trial Investigators and the australian and new zealand intensive care society clinical trials group: Decompressive craniectomy in diffuse traumatic brain injury. New England Journal of Medicine 2011; 364(16): 1493-1502.
- Stubbe H, Wölfler J: Traumatic brain injury in adults. Intensive care medicine up2date 2012; 8: 253-269.
InFo NEUROLOGY & PSYCHIATRY 2015; 13(2): 10-14.











