In recent years, the treatment spectrum for patients with inflammatory bowel disease (IBD) has expanded significantly. Based on the recognition that chronic and untreated inflammation adversely affects disease progression, early drug intervention and intensive monitoring are nowadays advocated to prevent complications.
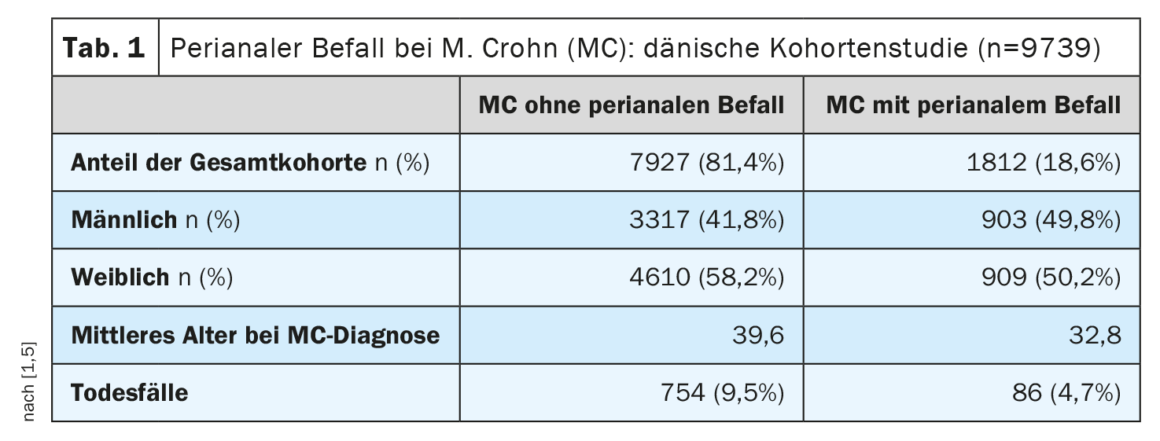
Crohn’s disease (MC) is characterized by a heterogeneous picture of different phenotypes [1]. The assessment of MC activity and severity is mainly based on laboratory parameters and clinical and anamnestic information (e.g., stool frequency, well-being, pain). These parameters have been summarized in various clinical scores, with the Crohn’s Disease Activity Index (CDAI) being the most common [2]. According to FDA/EMA, the cut-off value for moderate to severe MC is CDAI ≥220 [3]. Professor David T. Rubin, MD, University of Chicago, pointed out that patients with mild MC but perianal disease should also be classified as moderate to severe with regard to treatment. Perianal infestation occurs in approximately 20% of MC patients during the course of the disease [4,5]. This is one of the findings of a large-scale Danish cohort study (Table 1).
Monitoring of disease and treatment progression
Therapy is initially aimed at inducing remission. The long-term goal is to maintain remission. Disease control is understood as a multidimensional construct in MC (box) . The range of drug treatment options has expanded considerably. The introduction of tumor necrosis factor (TNF)-α antibodies was followed by the α4β7 antibody vedolizumab and the (IL)-12/ IL-23 antibody ustekinumab. It is important to assess the course of disease and treatment against the background of the time dimension, Prof. Rubin said. Several post-hoc analyses have shown that the response of MC patients with shorter disease duration to treatment with TNF-α antibodies, ustekinumab, and vedolizumab is better than those with longer disease duration [1,6,7].
| Evidence-based therapy decision The choice of therapy is based on a risk-benefit assessment, taking into account various factors and special treatment situations. In order to make evidence-based treatment decisions, an appropriate interpretation of the data is a prerequisite. Randomized-controlled trials are rated as strong empirical evidence, followed by head-to-head studies, post-marketing data, network metanalyses, real-world data, case reports, and finally expert opinion in descending order of evidence. |
For example, a meta-analysis published in 2020 including 18,471 patients showed that early use of biologics (ie. within two years of diagnosis) was associated with a two- to threefold increased likelihood of clinical remission at 6-12 months (OR 2.10 [95% CI: 1.69-2.60], n=2763, p<0.00001) and higher mucosal healing rates (OR 2.37 [95% CI: 1.78-3.16], n=994, p<0.00001) [8].
Vedolizumab and ustekinumab: current study data
In the SEAVUE study, ustekinumab and adalimumab were subjected to a head-to-head comparison [9]. This was a randomized, double-blind, active-controlled trial in a parallel-group design. In total, evaluable results were available from 386 biologics-naive patients treated with either ustekinumab (n=191) or adalimumab (n=195). The clinical endpoint at week 52 showed no significant differences between the two biologics: response rates for adalimumab and ustekinumab were 61.0% and 64.9%, respectively (p=0.417), with clinical remission operationalized as CDAI <150 at week 52. However, in terms of tolerability, ustekinumab performed slightly better.
In a secondary analysis published in 2022, ustekinumab was shown to be superior to vedolizumab in the second-line setting (after anti-TNF therapy). The analysis was based on 5 studies (4 retrospective and 1 prospective) in MC patients with failed anti-TNF treatment. In the maintenance phase, ustekinumab performed better than vedolizumab, and in the induction phase, the two monoclonal antibodies showed similar treatment effects [10].
Exhaust treatment options
Other biologics therapies are currently being investigated in pivotal trials, including, for example, risankizumab. This antibody binds to the p19 subunit of human interleukin-23 (IL-23), inhibiting interaction with its receptor. In clinical trials, risankizumab was shown to be effective in both first-line and biologics-experienced patients [11]. A good response to risankizumab was also seen in patients who had previously been treated with ustekinumab. According to the speaker, risankizumab is suitable in an early phase of treatment due to its good safety and tolerability. According to current data, use in later treatment sequences is also reasonable.
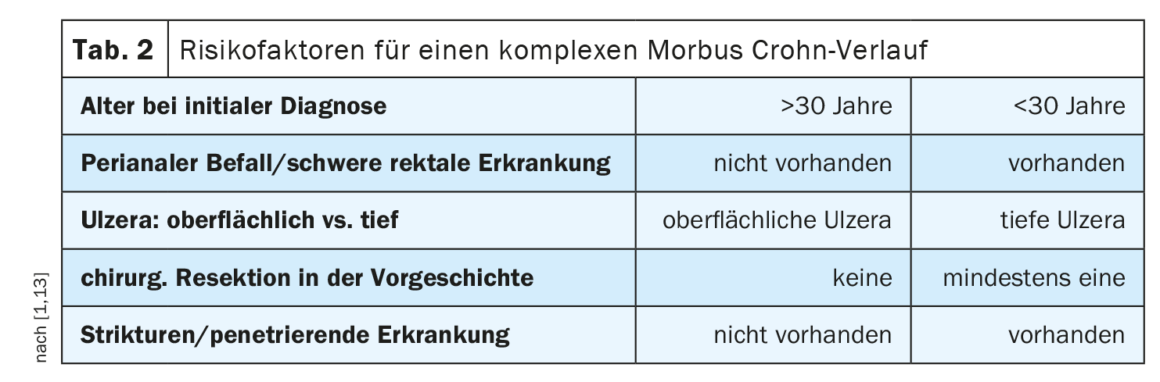
| Disease Control as a Multidimensional Construct Important patient-related factors for assessing disease control include BMI, gender, diet, pharmacogenomics, adherence, smoking. Disease-related factors include diagnosis latency, phenotype, extraintestinal factors, and previous treatment attempts, in addition to severity and disease activity. The therapy-related factors refer to the pharmacokinetic and pharmacodynamic properties of the agent, as well as the route of administration and the overall benefit-risk profile. Predictors of a complex course in MC in terms of independent risk factors at diagnosis (Table 2) included younger age, perianal manifestations, and the need for early steroid therapy. |
| according to [3] |
Regarding the sequence of biologics therapy and surgical intervention, current guideline recommendations advise not to discontinue biologics therapy in the long term, but rather to schedule surgery at the end of the normal administration interval of biologics, if possible, provided that the disease activity allows this [1].
In small molecules, the Janus kinase (JAK) inhibitor tofacitinib has so far only been approved for ulcerative colitis. Upadacitinib, also a JAK inhibitor, is currently being evaluated in phase III clinical trials for the treatment of Crohn’s disease [12].
Congress: European Crohns and Colitis Organization
Literature:
- “Diagnosis and Therapy of Crohn’s Disease,” German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS), updated S3 guideline, August 2021 – AWMF registry number: 021-004.
- Best WR, et al: Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439-444.
- “Treatment Sequencing in moderate-to-severe CD: Who’s first and who’s last?”, Prof. David T. Rubin, MD, ECCO, Copenhagen, March 1-4, 2023.
- Eglinton TW, et al: Clinical and genetic risk factors for perianal Crohn’s disease in a population-based cohort. Am J Gastroenterol 2012; 107: 589-596.
- Wewer MD, et al: The Incidence and Disease Course of Perianal Crohn’s Disease: A Danish Nationwide Cohort Study, 1997-2015. J Crohns Colitis 2020; 15: 5-13.
- Alric H, et al: The effectiveness of either ustekinumab or vedolizumab in 239 patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther 2020; 51: 948-957.
- Hamdeh S, et al: Early vs Late Use of Anti-TNFa Therapy in Adult Patients With Crohn Disease: A Systematic Review and Meta-Analysis. Inflamm Bowel Dis 2020; 26: 1808-1818.
- ngaro RC, et al: Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn’s disease. Aliment Pharmacol Ther 2020; 51(9): 831-842.
- Sands BE, et al: SEAVUE Study Group. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naive patients with moderately to severely active Crohn’s disease: a multicentre, randomised, double-blind, parallel-group, phase 3b trial. Lancet 2022; 399(10342): 2200-2211.
- Parrot L, et al: Systematic review with meta-analysis: the effectiveness of either ustekinumab or vedolizumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor. Aliment Pharmacol Ther 2022; 55(4): 380-388.
- Farrante M, et al: Presented at UEGW. Oct 2022. op128.
- Chugh R, et al: Multicentre Real-world Experience of Upadacitinib in the Treatment of Crohn’s Disease. J Crohns Colitis 2023; 17(4): 504-512.
- (ACG) Lichtenstein GR, et al: ACG Clinical Guidelines: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113: 481-517.
HAUSARZT PRAXIS 2023; 18(5): 37-38 (published 5/25-23, ahead of print).