Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP1-RA) play an important role in diabetes therapy. However, their effect on diabetic neuropathy and retinopathy is not clear. In a real-world study, British scientists investigated the relationship between SGLT2i and GLP1-RA and the 5-year risk of diabetic or autonomic neuropathy, diabetic retinopathy and macular edema, hospitalization and all-cause mortality in type 2 diabetes.
In the treatment of diabetic neuropathy, there is a lack of therapeutic strategies that target the underlying mechanism of nerve damage. Instead, the treatment is limited to symptomatic treatment with neuropathic painkillers, explained Aikaterini Eleftheriadou, Department of Cardiovascular and Metabolic Medicine, University of Liverpool, at the beginning of her presentation [1].
There are a number of treatment options available for diabetic retinopathy, but these are aimed at the progression of the disease. These include: Anti-VEGF injections and intravitreal steroid injections, which can be costly. There is also a group of patients who do not respond to anti-VEGF injections. Laser photocoagulation is another option, but it essentially triggers a destructive process.
“We should therefore try to prevent these diseases from occurring in the first place or halt their progression once they have occurred.” The expert cited several studies in which the role of intensive blood glucose control and multifactorial management was investigated:
- The Steno-2 study showed that the risk of retinopathy and autonomic neuropathy was lower in the group with intensive glycemic control.
- However, the ADVANCE study, the largest of these studies, showed no significant effects on retinopathy.
- In the ACCORD study, a delayed onset of eye complications and neuropathy was observed in some areas.
- The UKPDS-33 study showed a lower rate of necessary photocoagulation.
In terms of drug treatment, data show that angiotensin-converting enzyme inhibitors (ACEi) can reverse diabetic retinopathy and angiotensin receptor blockers (ARBs) can reduce the improvement of retinopathy in patients who already had retinopathy at baseline.
Contradictory data to date
Regarding specific medications for diabetics, some data suggest that SGLT2 inhibitors may have beneficial effects on indices of autonomic neuropathy and may be neuroprotective. However, the CREDENCE study, a post-hoc analysis of an RCT with 4401 participants, showed that canagliflozin had no effect on the risk of neuropathy events.
As far as retinal disease is concerned, according to Aikaterini Eleftheriadou, there is also a current meta-analysis that shows a positive influence of SGLT2i, while older meta-analyses showed no influence on this disease.
For glucagon-like peptide-1 receptor agonists (GLP1-RA), most data show no effect on neuropathic outcomes and retinal disease, with the exception of the Qatar study, in which exenatide and pioglitazone were associated with corneal nerve regeneration. In contrast, the SUSTAIN-6 study showed an increased risk of retinopathy with semaglutide, although this study was primarily focused on cardiovascular outcomes.
| Restrictions |
| Aikaterini Eleftheriadou pointed out the limitations of the study: the data were retrospective data from practice, which is why randomization and control were not possible. In addition, the data was extracted from electronic health records, which can lead to possible incompleteness. In addition, the results were based on ICD-10 coding – neuropathy data could therefore be biased. The results of their investigation should therefore lead to further research. Eleftheriadou therefore recommended dedicated microvascular disease studies to investigate the effect of SGLT2i on diabetic neuropathy and eye disease in patients with type 2 diabetes. In particular, 2 questions should be investigated: |
| 1. At what point in the course of the disease should SGLT2i be started? |
| 2. When conducting studies: Which study endpoint should be set specifically with regard to neuropathy? |
Large-scale real-world study
For their real-world study, Eleftheriadou and colleagues used data from the international research network TriNetX. The aim was to evaluate the effect of SGLT2i and GLP1-RA on diabetic neuropathy and eye disease. Hospital admissions due to heart failure were also included as a positive control result.
Their retrospective cohort analysis included information from six million people with type 2 diabetes in 85 healthcare organizations. Two intervention cohorts (SGLT2i, n=126 171; GLP1-RA, n=164 024) were compared with a control cohort (no SGLT2i/GLP1-RA, n=1 665 412). Cohorts were matched 1:1 by propensity score matching for age, sex, ischemic heart disease, microvascular complications, HbA1c <7% and 7%, BMI, eGFR, and use of relevant medications (ACEi, lipid-modifying agents, metformin, pioglitazone):
- SGLT2i vs. control: 141 ‘236 patients in each cohort
- GLP1-RA vs. control: 160,970 patients in each cohort
- GLP1-RA vs. SGLT2i: 125,383 patients in each cohort.
In addition, a sub-analysis was carried out to compare the two intervention cohorts. The main outcomes were diabetic neuropathy, autonomic neuropathy, retinopathy and macular edema, and the outcome definition was based on ICD-10 coding. “We started our time window about 20 years ago, in September 2003. We defined the index event as the start of insulin treatment in the control group, the start of both SGLT2i treatment and insulin treatment in the SGLT2i cohort, and the start of both GLP1-RA and insulin treatment in the GLP1-RA cohort.” The data were collected 5 years after the index event, the time window was closed in September 2023.
Data speak for the use of SGLT2i
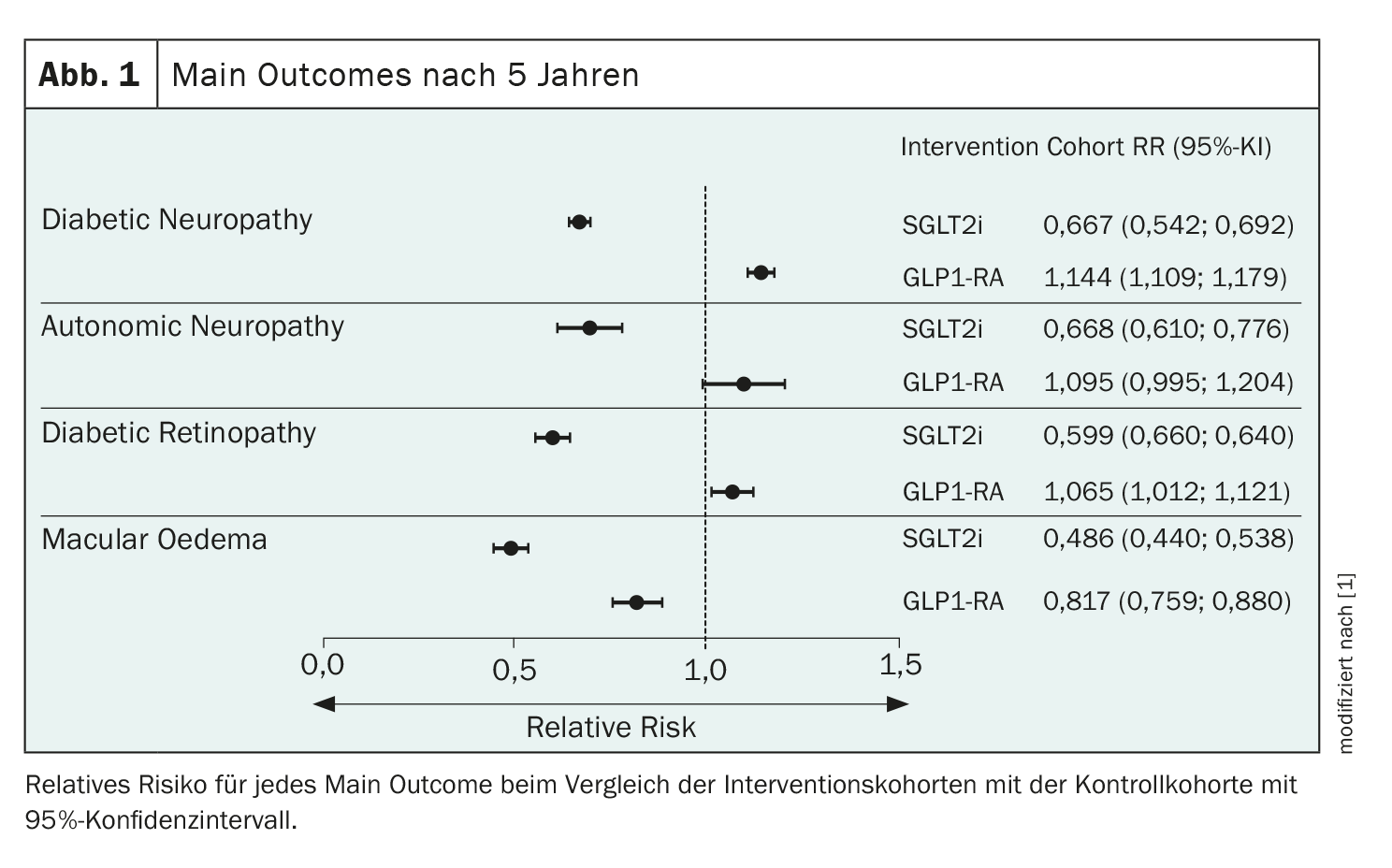
The main outcome was relative risk after five years for SGLT2i and GLP1-RA. SGLT2i significantly reduced the risk of diabetic neuropathy, autonomic neuropathy, diabetic retinopathy and macular edema (Fig. 1). GLP1-RA was associated with a slight increase in the risk of diabetic neuropathy and a smaller, non-significant increase in the risk of autonomic neuropathy and diabetic retinopathy. However, this also reduced the relative risk of macular edema.
Both SGLT2i and GLP1-RA showed a significant relative risk reduction in heart failure, hospitalization and all-cause mortality. A direct analysis between the GLP1-RA and the SGLT2i cohort after 5 years showed that GLP1-RA is associated with an increased risk in direct comparison to SGLT2i.
“Our data demonstrate the real-world benefit of SGLT2 inhibitors on neuropathy, autonomic neuropathy, retinopathy and macular edema in patients with type 2 diabetes treated with insulin,” concluded Aikaterini Eleftheriadou. GLP1-RA showed an increased risk for neuropathy and a lower risk for autonomic neuropathy and retinopathy, but proved to be protective for macular edema. SGLT2i showed better results in microvascular complications in direct comparison with GLP1-RA: SGLT2i therapy was associated with the greatest risk reduction in diabetic microvascular complications as well as heart failure, hospitalization and all-cause mortality. “So there is a clear benefit in all events analyzed, and these data further support the use of SGLT2i in our patients with type 2 diabetes.” Future randomized controlled trials of SGLT2i and GLP1-RA should include sensitive surrogate biomarkers of diabetic microvascular disease to validate these results. According to the expert, SGLT2i in particular should generally be ranked higher in the treatment algorithm for patients with type 2 diabetes.
Source:
- Eleftheriadou A: Presentation “Risk of diabetic microvascular complications, heart failure, hospitalization and all-cause mortality with SGLT2i and GLP1-ra in type 2 diabetes: a real-world data study”; EASD Congress 2023, Hamburg, 4.10.2023.
Congress: EASD 2023
InFo DIABETOLOGY & ENDOCRINOLOGY 2024; 1(1): 32-33 (published on 12.2.24, ahead of print)