Urothelial carcinoma accounts for 90% of urinary bladder tumors. Every year, 1250 people in Switzerland contract the disease – predominantly older men. Standard treatment is cisplatin-containing chemotherapy. Immunotherapy is playing an increasingly important role in metastatic forms.
Urothelial carcinoma accounts for 90% of urinary bladder tumors. In Western countries, squamous cell carcinoma, adenocarcinoma, and small cell carcinoma are rare. Sarcomas, lymphomas or melanomas are even rarer. Urothelial carcinoma of the urinary bladder is responsible for about 3% of cancer cases in Switzerland, i.e. every year 1250 new cases are diagnosed [1]. 75% of those affected are men. More than 60% are over 70 years of age at the time of diagnosis. Risk factors are nicotine and pollutants such as aromatic amines (occupational disease), st. n. chemotherapy and radiation therapy. Rarely, urothelial carcinoma of the upper urothelial tract occurs in the setting of Lynch syndrome. Patients often present with macrohematuria, pollakiuria, dysuria, and urinary urgency symptoms at diagnosis. Unfortunately, 20-25% are already muscle invasive at initial manifestation. Patients with metastases historically have a median survival of only 12-18 months.
The localized stage
The common early stages (non-muscle invasive urothelial carcinomas) are radically resected by transurethral resection (TUR bladder). Patients at intermediate or high risk are subsequently treated with intravesical therapy with BCG or chemotherapy. Patients who do not respond to BCG now have the option of being treated with immunotherapy. In the phase 2 trial (Keynote-057 trial), patients with high-risk, non-muscle-invasive bladder cancer in non-response to BCG were treated with pembrolizumab every 3 weeks for 2 years. A complete remission rate of 41% was achieved. Side effects are comparable to those in the metastatic setting. The therapy was approved by the FDA in the USA [2].
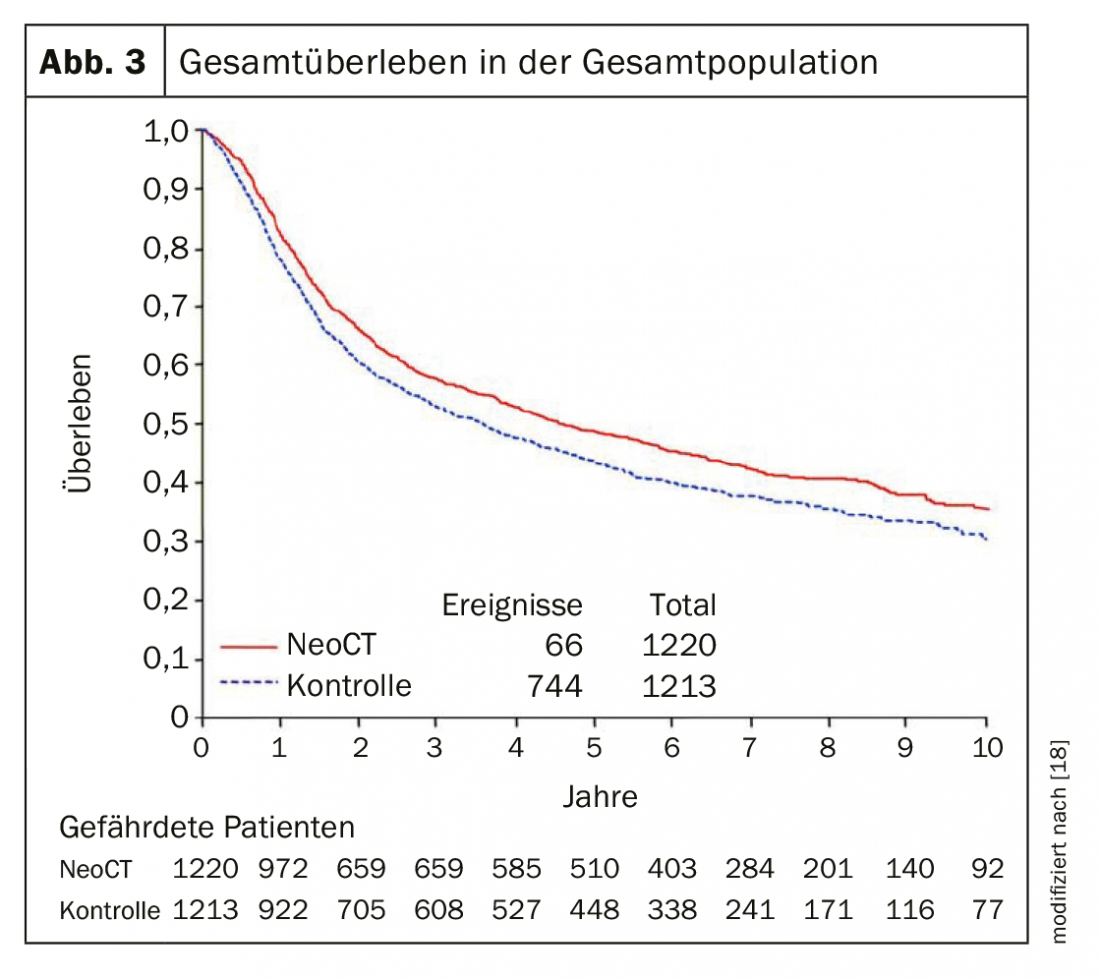
Muscle-invasive urothelial carcinoma in the localized stage remains a major problem. Approximately 50% of patients with muscle-invasive bladder cancer die within 3 years due to metastasis despite cystectomy. Therefore, from stage T2 or N+, patients should be offered neoadjuvant chemotherapy (NAC). With 3-4 cycles of cisplatin-containing chemotherapy (cisplatin and gemcitabine or dose-dense MVAC), 5-year survival was increased by an absolute 5% (Fig. 1). A meta-analysis showed pathologic downstaging (<pT2) in 49% of patients by NAC with cisplatin and gemcitabine. After NAC, the postoperative rate of ypT0-N0 tumors was 25-30%, which has a significant impact on progression-free and overall survival [3]. Unfortunately, the acceptance of neoadjuvant chemotherapy has increased only slightly in recent years. Only a subset of patients will receive neoadjuvant chemotherapy. In addition, there are patients who are not chemotherapy-eligible due to their co-morbidity, i.e., chemotherapy with cisplatin is not an option for them. Replacement of cisplatin with carboplatin is associated with a significantly worse outcome and thus should not be performed. The good results of immunotherapy in the metastatic setting has led to the addition of immunotherapy to neoadjuvant chemotherapy currently being evaluated in several trials. Phase 2 studies demonstrated that neoadjuvant therapy with the checkpoint inhibitors atezolizumab and pembrolizumab can achieve a complete pathologic response rate of 30-40%, and the overall response rate (<pT2) was 40-50% [4,5]. Crucially, PDL-1 expression was associated with a better response rate.
The results of the BLASST-1 trial, which evaluated nivolumab in combination with cisplatin and gemcitabine, showed a complete pathologic remission of 49% [6]. With neoadjuvant chemotherapy with cisplatin/gemcitabine alone, a pCR of about 30% is found. The SAKK 06/17 trial, which is evaluating neoadjuvant chemotherapy with cisplatin and gemcitabine in combination with the immunotherapy durvalumab followed by postoperative maintenance therapy with durvalumab, was recently closed. Interim analysis showed a pathologically complete remission rate of 30%. The final results are eagerly awaited.
The gold standard for treatment of muscle-invasive urothelial carcinoma from stages T2-4a, N0-X M0 is still radical cystectomy and pelvic lymphadenectomy (with ileum conduit or replacement bladder). In high-risk situations such as recurrence after BCG instillation therapy or extensive or multilocular high-grade tumors, early cystectomy is recommended starting at stage pT1. Timing of surgery after NAC is important. When the interval between chemotherapy and cystectomy exceeds 10 weeks, overall survival becomes significantly worse. Also, foregoing surgery for clinically complete response is negligent, as residual tumor is present in 64% after cystectomy has been performed. The proportion of pT3/4 urothelial carcinomas with and without lymph node involvement was 25%.
Bladder-preserving “trimodal therapy” consisting of a combination with radical transurethral resection as well as radio-chemotherapy may be an alternative for inoperable patients or patients with a strong desire for organ preservation, but shows a worse oncologic outcome [7].
Adjuvant chemotherapy should be considered if neoadjuvant chemotherapy has not been performed. Especially from stages T3 and N+. Data from meta-analyses on this are less robust, but it suggests that these patients also benefit [8].
Metastatic stages
Patients with relapsed or metastatic disease have a very poor prognosis (12-18 months). Prognostic factors particularly unfavorable in terms of overall survival are: poor general condition with a Karnofsky Performance Status <80% and visceral metastases including skeletal metastases. Standard chemotherapy with cisplatin and gemcitabine results in a 50% response [9]. The median survival is 14 months. After all, 13-15% of patients are still alive after 5 years. In patients with good general condition and absent visceral metastases, the median survival is as high as 33 months and 20% are still alive after 5 years. Unfortunately, this therapy is not feasible in many elderly patients due to co-morbidity. In clinical practice, carboplatin is often used instead of cisplatin, which is much less effective in urothelial carcinoma.
With the checkpoint inhibitors, there is now a new therapeutic option, which offers a possibility of treatment, especially for older patients who are not cisplatin-competent. In the phase 2 Keynote-052 study, patients were treated with pembrolizumab 200 mg every 3 weeks for up to 2 years [10]. This showed a 29% response including 7% complete remission. Response was most evident with PD-L1 expression above 10% (38%), although response was also seen with PD-L1 expression below 10%. In the phase 2 IMvigor 210 trial, atezolizumab achieved a median survival of approximately 16 months [11]. Patients who had strong PD-L1 expression benefited significantly better from immunotherapy (median survival of 19 months). Response was 32% with 9% complete remissions.
In the 2nd lines after failure of platinum-containing therapy, one can expect a response of about 10-20%. Vinflunine was tested in a phase 3 trial against best supportive care [12]. There was a 9% response and a benefit in terms of median survival (6.9 versus 4.6 months).
In IMvigor 211, a phase 3 trial comparing atezolizumab with various chemotherapeutic agents, no significant effect of immunotherapy was seen [13]. However, the phase 3 Keynote-045 trial, which also compared immunotherapy (pembrolizumab) with various therapies (vinflunine, taxanes), was positive, with survival of 10.3 vs 7.4 months. After one year, 44% versus 33% of patients receiving chemotherapy were still alive [14].
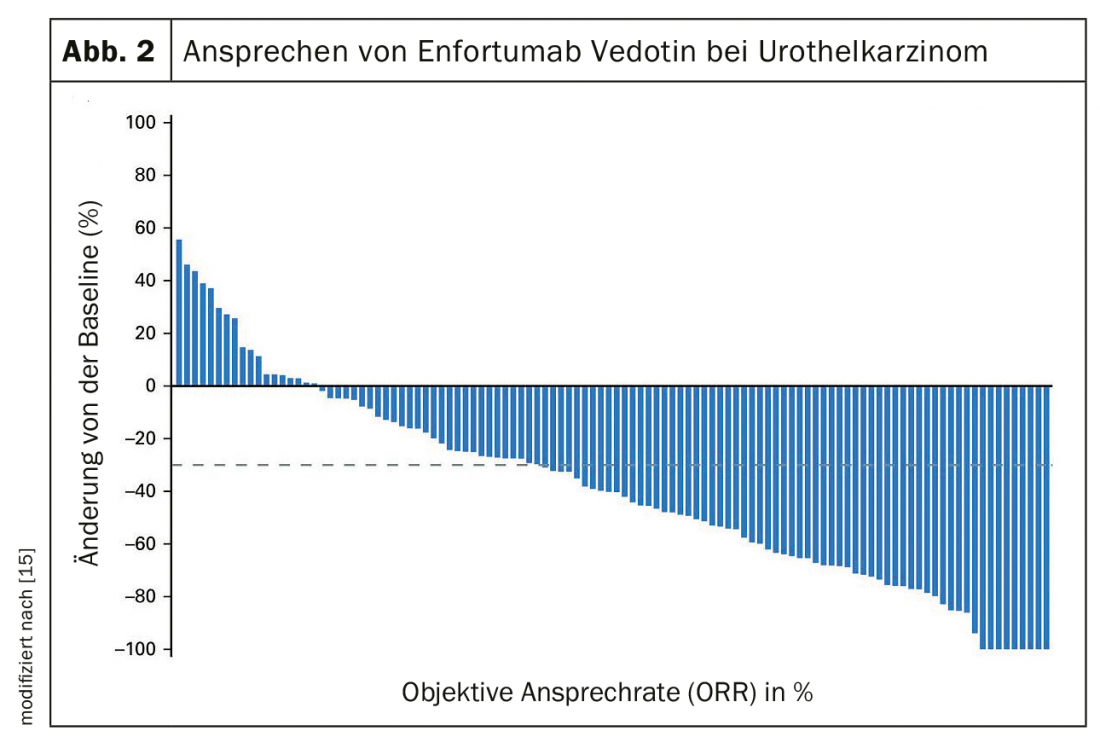
After failure of platinum-containing chemotherapy and immunotherapy, therapy with enfortumab vedotin, an antibody-drug conjugate, may be considered [15]. The treatment response in the phase 2 study was still 44% with 12% complete remissions (Fig. 2) . The median response was 7.6 months.
For patients with an FGFR 3 gene mutation or an FGFR 2 or 3 gene fusion, therapy with the FGFR inhibitor Erdafitinib is an option. In an open-label phase 2 trial, patients with at least one prior therapy were treated with Erdafitinib [16]. There was a response in 40% and a progression-free and overall survival of 5.5 resp. 13.8 months. Approval in the U.S. was based on response rate. The therapy is now being tested against chemotherapy and immunotherapy in a phase 3 trial.
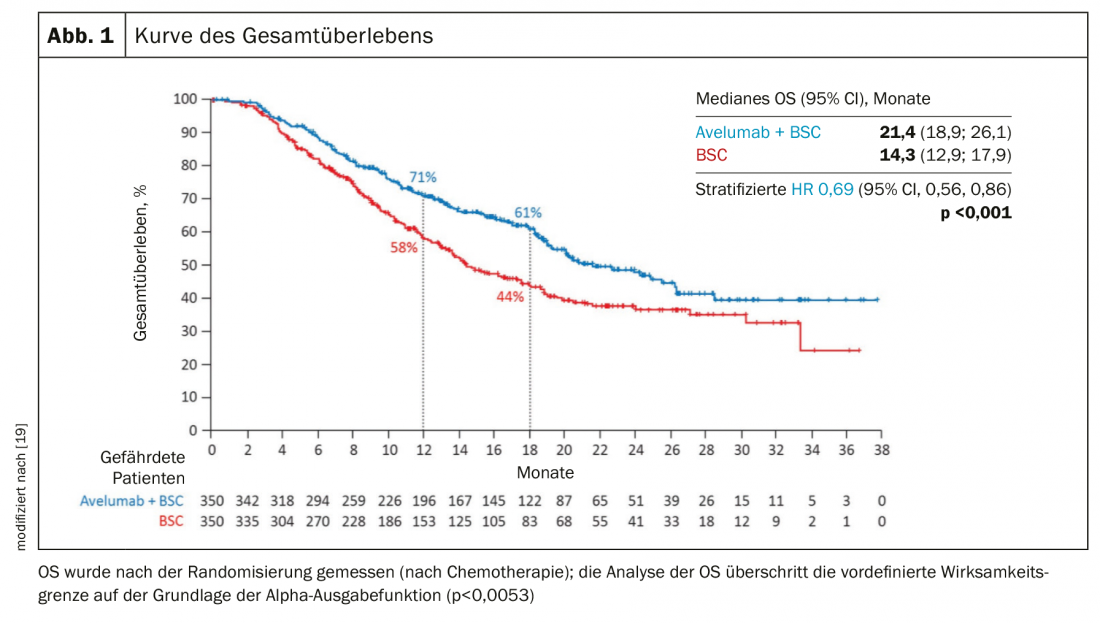
Whether primary chemo-immunotherapy, which is already standard in bronchial carcinoma, is also an advantage in urothelial carcinoma is currently unclear. Due to the high “tumor mutational load”, which is an indication for a good response to immunotherapy in many tumors, an advantage of an early use of immunotherapy is plausible. IMvigor130, a phase 3 trial comparing platinum/gemcitabine +/- atezolizumab, was negative with respect to co-primary endpoint overall survival [17]. In contrast, progression-free survival was 1.9 months better (6.3 vs 8.2 months). In the Phase 3 JAVELIN Bladder 100 trial, patients who were not progressive after cisplatin-containing chemotherapy were treated with immunotherapy with avelumab. Patients with avelumab lived an average of 7 months longer (OS 14.3 vs 21.4 months) (Fig. 3) [18].
Take-Home Messages
- Neoadjuvant cisplatin-containing chemotherapy remains standard in localized tumor from stage T2 and improves survival by an absolute 5%.
- In metastatic urothelial carcinoma, maintenance therapy with the checkpoint inhibitor avelumab improves survival by 7 months in response to platinum-containing chemotherapy.
- In elderly and polymorbid patients with locally advanced and metastatic urothelial carcinoma, the option of immunotherapy alone is well tolerated.
- Molecular markers are also of increasing importance in the treatment of urothelial carcinoma with the option of “targeted therapy”, e.g. with the FGFR inhibitor Erdafitinib.
- Therapy with the antibody-drug conjugate enfortumab vedotin has a high response rate even in pretreated patients.
Literature:
- www.nicer.org
- www.fda.org/drugs
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis for individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005; 48(2): 202-205.
- Powles T, et al: A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). J Clin Oncol.2018;36(suppl 15; abstr 4506).
- Necchi A. et al: Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018;36(34): 3353-3360.
- Gupta S, et al: Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J Clin Oncol 2020;38(suppl 6; abstr 439).
- Chedgy ECP, et al: Radical cystectomy and the multidisciplinary management of muscle-invasive bladder cancer. JAMA Oncol 2016;2(7):855-856.
- Leow JJ et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014 Jul;66(1):42-54.
- Van der Maase H, et al: Long-term survival results of randomized trial comparing gemcitabine plus cisplatin with methotrexate, vinblastine, doxorubicin plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23(21): 4602-4608.
- Balar AV, et al: First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11): 1483.
- Balar AV, et al: Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389(10064): 67.
- Bellmunt J, et al: Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27): 4454.
- Powles T, et al: Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018; 391: 748-757.
- Bellmunt J, et al: Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11): 1015.
- Rosenberg JE et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019;37(29):2592.
- Loriot Y et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381(4): 338
- IMvigor 130. ESMO 2019.
- Powles, et al: Javelin Bladder 100. ASCO 2020. journal of clinical oncology 38, no. 18_suppl; DOI: 10.1200/JCO.2020.38.18_suppl.LBA1.
- Vale CL: Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and metaanalysis. European Urology 2005.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(4): 6-9.