Echinacea purpurea (L Moench), the coneflower, is one of the most widely used medicinal plants. Numerous clinical studies document the efficacy of Echinacea for the treatment and prevention of flu-like infections. The following article presents a new meta-analysis demonstrating that preventive treatment with echinacea significantly reduces the risk of recurrent respiratory tract infections.
As soon as the cool season begins, thousands of people again suffer from a viral cold. The result is fever, cold and often cough. Sufferers seek advice from a doctor or pharmacy and request appropriate medication. Many of them do not want to or cannot interrupt their work and therefore keep themselves “afloat” with medication until the flu-like infection is over.
Among the medicines that reduce fever, headache and pain in the limbs, decongest the nasal mucous membranes and have an antitussive or secretolytic effect, the medicinal plant Echinacea from North America occupies a special position. It is suitable both to fight an existing viral infection and to strengthen the immune system. As a result of such immune modulation, the corresponding individuals are less susceptible to influenza infections. A recent meta-analysis shows that echinacea also reduces the risk of recurrences and complications such as pneumonia, otitis media/externa, or tonsillitis/pharyngitis [1].
Such infections run with a decrease in salivary gland immunoglobulin (Ig) A as well as interferon-gamma (IFN-gamma). These two substances usually confer immunity against recurrences. Untreated infection often leads to recurrence and complications. [2–4].
Echinacea extracts could offer an interesting solution here. Recent studies show an immunomodulatory effect by interacting with endocanabinoid receptors. Further, they led to a decrease in TNF-alpha (tumor necrosis factor-alpha), as well as an increase in IFN-gamma [5,6].
Meta-analysis
The meta-analysis discussed here evaluated studies that provided results on the risk of recurrent respiratory tract infections (RTI) and complications after treatment with echinacea.
To this end, a literature search was conducted and randomized, placebo-controlled human trials were sought for meta-analysis that reported recurrent respiratory tract infections in subjects without composite allergy who were treated with echinacea. All studies found were reviewed. Studies whose Jadad score was not at least 4 were not included.
Of the original 101 studies, 89 did not meet requirements because end points were unusable or did not capture recurrences. Of the 12 remaining studies, one had a Jadad score of 3 and was therefore not included. Three studies worked with induced infections, and two other studies were subanalyses of previously included studies. The remaining six studies were included in the analysis (Table 1).
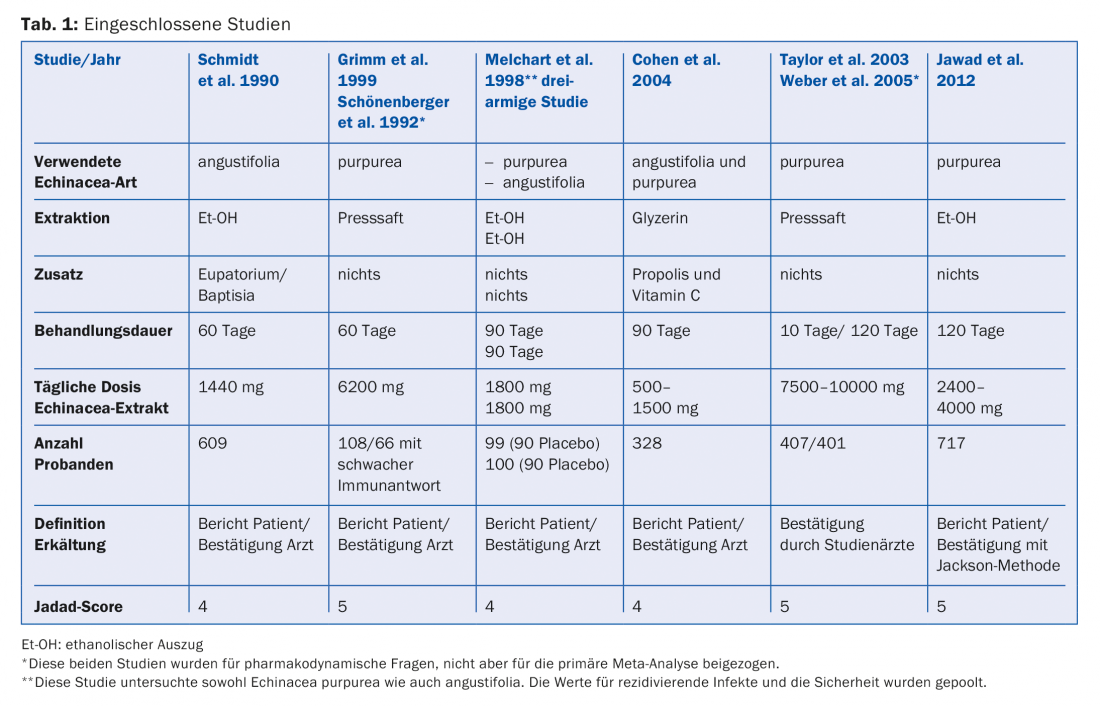
Primary endpoint
The primary endpoint of the evaluation was recurrent infection risk, that is, the total number of influenza infections that occurred during two to four months of treatment with echinacea or placebo, or in one study during the four-month surveillance period. Furthermore, the number of recurrent infections experienced by the subjects was recorded.
This evaluation included data of respiratory tract infections and subsequent complications that followed each 10-day treatment with echinacea. Safety was also assessed with the number of adverse events (AE) observed.
Results
Recurrent RTI: All studies reported a lower incidence of RTI in the echinacea groups compared with the placebo groups. But only in the studies of Cohen and of Jawad these values reached significance (p<0.0001 (Cohen) and p=0.009 (Jawad), respectively. When all studies were pooled, this resulted in a significantly lower incidence (p<0.0001).
Number of patients with ≥1 recurrent RTI: Four studies provided corresponding figures. The protective factor of Echinacea also reached significance here (p=0.041). In the study by Melchart et al. a slightly better result was obtained for E. pupurea than for E. angustifolia. However, because of the small number of subjects, the result should be taken with caution.
Subgroup evaluation: In a subgroup evaluation, the studies that used a lipophilic echinacea extract (Schmidt et al., Cohen et al., Jawad et al., Melchart et al.) and the studies that used a pressed juice (Grimm et al., Taylor et al.) were evaluated separately. This subgroup evaluation showed a significant reduction for recurrent infections for the ethanolic Echinacea extracts (p<0.0001). No significant reduction could be calculated for the two studies with pressed juice (p=0.283).
Complications: Preventive treatment with echinacea also achieved a significant reduction in RTI-related complications compared to placebo in the total analysis (p<0.0001). Among individual complications, this was also true for pneumonia (p<0.0001), tonsillitis/pharyngitis (p=0.0201), and otitis medi-a/interna (p <0.0001). For conjunctivitis (p=0.676), sinusitis (p=0.768), and bronchitis, the improvement did not reach significance. Treatment of such complications with antibiotics was reduced by up to 50% compared with placebo groups.
Safety: The safety profile of Echinacea proved to be very good. Most documented AEs proved to be mild. No significant difference was found between the number of AEs reported in the echinacea and placebo groups. Laboratory parameters remained stable, and the general condition of the patients remained generally (very) good in both groups.
Summary
The meta-analysis presented documents the preventive efficacy of echinacea for the long-term treatment of recurrent RTI and complications caused by it. Specifically benefit individuals at increased risk for RTI. Differences in efficacy can be attributed to the different manufacturing methods of echinacea extracts.
The safety profile of Echinacea is very good. Therefore, preventive treatment with echinacea is an effective therapy to reduce RTI recurrences and related complications. Treatments with antibiotics can also be reduced in this way.
Literature:
- Schapowal A, et al: Echinacea reduces the Risk of Reccurent Respiratory Tract Infections and Complications: A Meta-Analysis of Randomized Controlled Trials. Adv Ther 2015; 32: 187-200.
- Pene F, et al: Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003; 37: 929-932.
- Message SD, et al: Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004; 75: 5-17.
- Fox JP, et al: The Seattle virus watch. V. Epidemiologic observations of rhinovirus infections, 1965-1969, in families with young children. Am J Epi-demiol. 1975; 101: 122-143.
- Gertsch J, et al: Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004; 577(3): 563-569.
- Ritchie MR, et al: Effects of Echinaforce® treatment on ex vivo-stimulated blood cells. Phytomedicine 2011; 18: 826-831.
HAUSARZT PRAXIS 2016; 11(10): 2-4