Preoperative anemia is an independent predictor of increased risk of infection, thromboembolism, and mortality. For their part, allogeneic blood transfusions further contribute to worsening outcomes through nonspecific and specific risks. An algorithm-based approach to anemia is helpful. Based on EC cell volume (MCV) and reticulocytes, mild to moderate anemias can be classified and treated in the perioperative setting. Severe anemias should be evaluated and managed by an expert. In the case of expected high blood loss in the course of major surgery, the iron requirement can be estimated and already substituted preoperatively. According to current data, anemia is considered a contraindication for elective surgery.
The prevalence of anemia in the average population is between 10 and 30% worldwide, and as high as 60% in Africa and India. In the preoperative collective, an average of 39% of patients are anemic [1].
It is evidence-based medicine and now widely accepted that anemia is associated with multifaceted risks [2]: Preoperative anemia is an independent predictor of increased risk of infection and thromboembolism (TE) [3], increased mortality, and prolonged hospitalization [4]. Anemia is therefore a serious and relevant risk factor for the healing outcome [5] of patients undergoing elective surgery and must be treated. Despite the clear literature, the problem of preoperative or perioperative anemia is nowadays still very often “taken care of” by the administration of allogeneic blood transfusions with all the additional specific and nonspecific risks associated with it [6].
The Patient Blood Management (PBM) clinical concept is the patient-centered, interdisciplinary, and evidence-based response as a modern approach to anemia therapy that shifts the focus away from blood transfusion to the use of the patient’s own resources. PBM is built on three pillars (Table 1), of which the first pillar in particular consists of optimization of red blood cell volume in the preoperative and perioperative setting. This involves identifying anemia as a symptom, diagnosing the underlying disease and, as far as possible, treating it. Untreated anemia is now considered a contraindication for elective surgery.
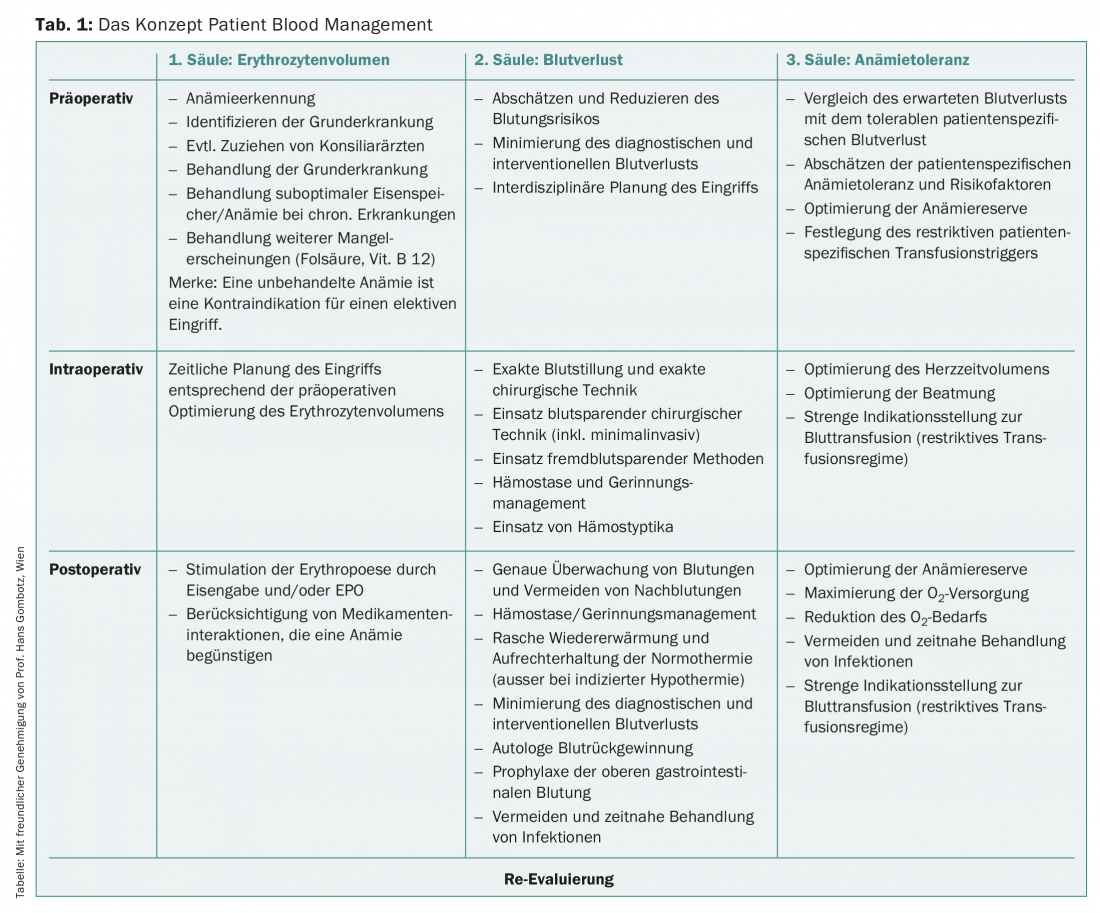
Clarification and therapy algorithm
An algorithm is enormously helpful in this situation because it can take into account the site-specific characteristics of the treating physician, such as accessibility of specialists and availability of complementary laboratory tests. With a clarification and therapy algorithm (Fig. 1 and Tab. 2), the majority of preoperative anemias can be diagnosed, classified, and treated in a standard population. As a consequence, patients show up on time and optimally prepared for the scheduled surgery.
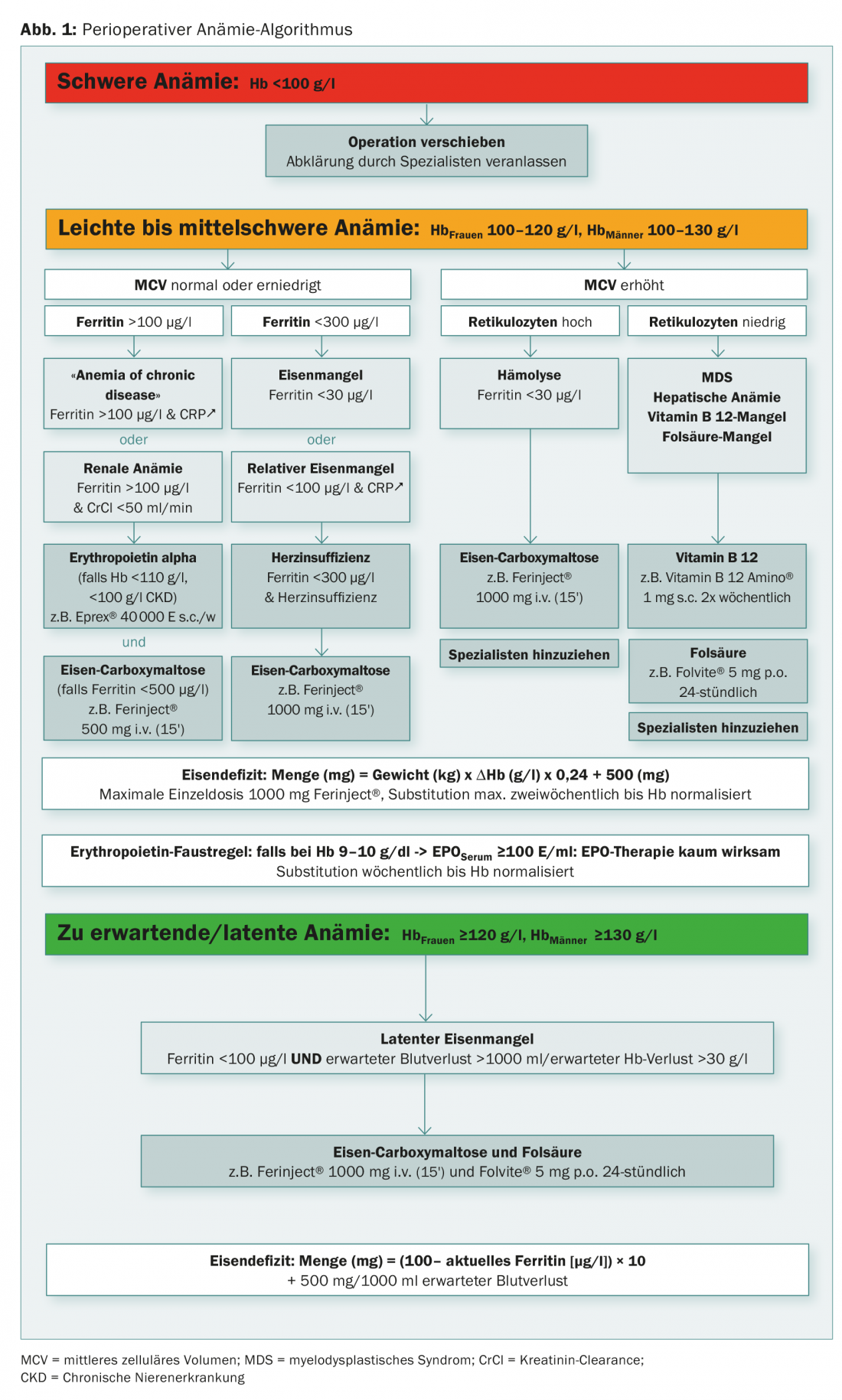
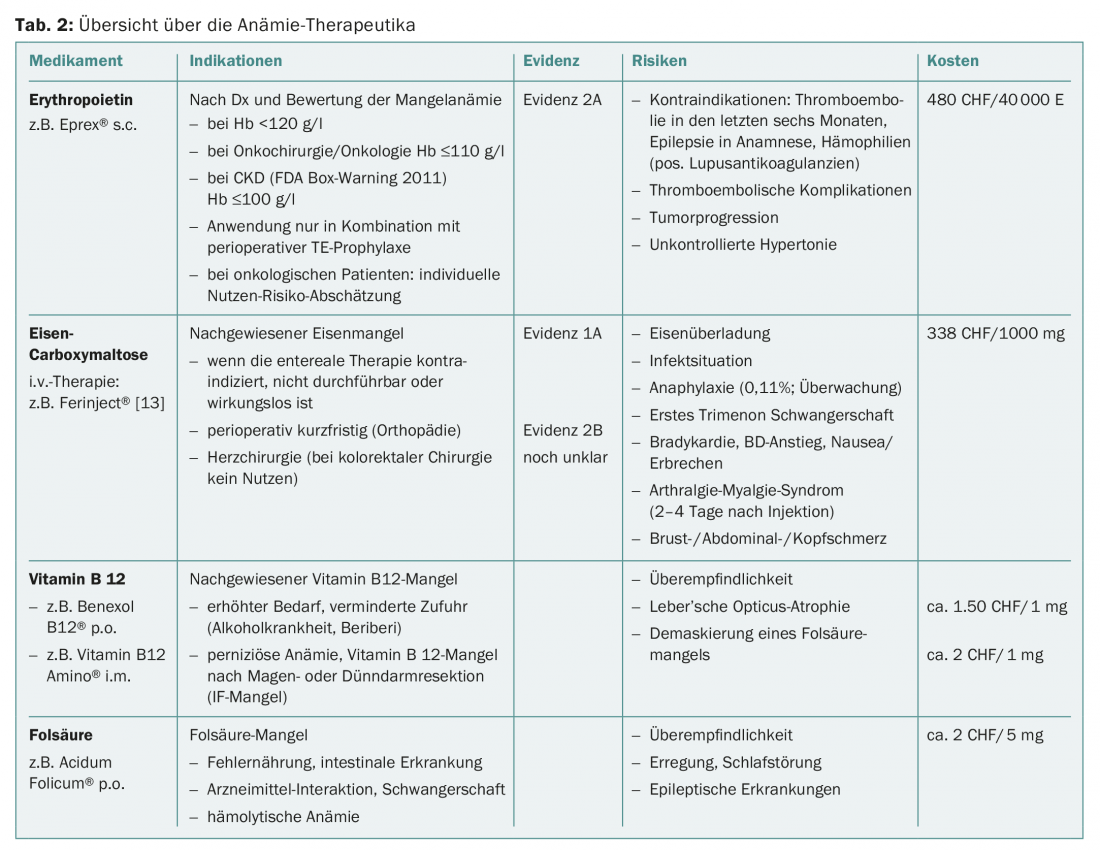
The greatest challenge is and remains the balancing act between medical correctness on the one hand and conciseness or simplicity on the other.
Infectious, gastrointestinal, and renal causes play a minor role worldwide, while iron deficiency is the causative agent of anemia in about 65% of the studied collective. Women and men are equally affected [7].
With increasing age, gastrointestinal and renal forms of anemia [8] increase and the caring physician is often confronted with mixed forms, the diagnosis of which is more demanding. However, these anemias are also substantially associated with iron deficiency, which remains the most common cause in old age [7]. In the preoperative context, iron deficiency also occurs due to impaired iron utilization in the context of inflammatory syndromes or malignancies.
Following the WHO definition of anemia, we define three severity levels in our algorithm:
- severe anemia (Hb <100 g/l)
- mild to moderate anemia (Hb 100-120 or 130 g/l)
- latent or expected anemia (Hb >120 or 130 g/l).
Severe anemia
In case of severe anemia <100 g/l, the planned surgery should be postponed and the patient should be evaluated by a specialist.
Mild to moderate anemia
According to WHO, mild to moderate anemia is defined by Hb level of 100-120 g/l in women and 100-130 g/l in men.
In this anemia, we are primarily guided by mean cellular volume (MCV). In most cases of normal or decreased MCV, a manifest or hidden iron deficiency is present (Fig. 2A). With ferritin above 100 μg/l with elevated CRP in terms of “anemia of chronic disease” or with impaired renal function in terms of “renal anemia”, we recommend a combination therapy of erythropoietin (Epo) and iron in the dosage indicated in the algorithm. The ideal frequency and dosage of Epo therapy have not been established. Three days after a first dose, reticulocytes increase, and after seven days the blood volume of one EC concentrate is generated (after 28 days of five EC concentrates). In the case of a ferritin value below 30 μg/l or a value below 100 μg/l in combination with signs of inflammation, we recommend the isolated substitution of iron in the sense of an absolute or relative iron deficiency.
Figure 2B shows the normal reference between the two pathological blood smears.
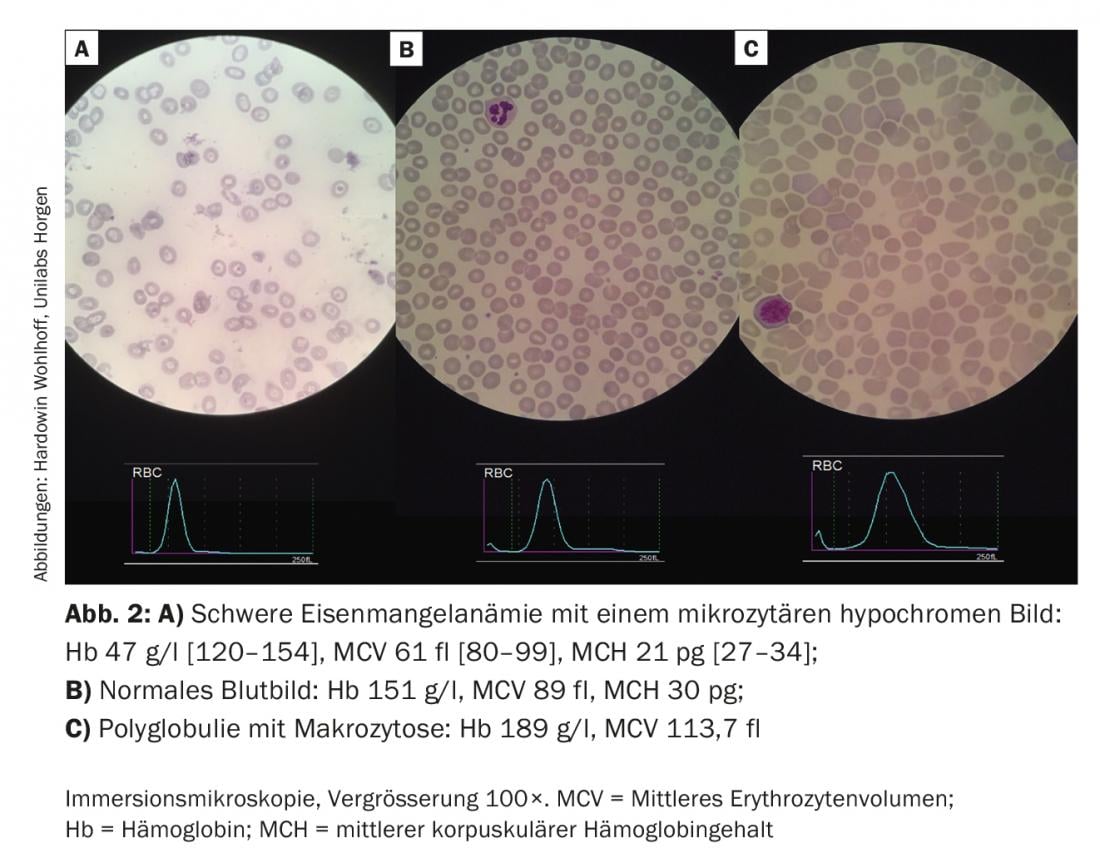
In macrocytic anemias (Fig. 2C), reticulocytes provide information on whether the anemia is hyper- or hyporegenerative.
Hyporegenerative anemias are usually deficiency-related (iron, folic acid, vitamin B 12). Less commonly, they indicate bone marrow insufficiency. Hyperregenerative anemias are the result of increased (hemolysis) and/or impaired production (myeloproliferative neoplasms, thalassemias).
We recommend that clarification and substitution of “simple” iron deficiency be done by primary care and referring physicians. In the case of more complex forms of anemia and treatment concepts, the involvement of a hematologist may be helpful.
In principle, iron therapy can be administered enterally if gastrointestinal tolerance is given and there is sufficient lead time. Nevertheless, data show that parenteral iron supplementation is associated with higher compliance and results in an earlier and more pronounced hemoglobin rise [9–14].
Similarly, the role of hepcidin as a key site of systemic iron regulation or iron absorption is discussed in the literature: On the one hand, iron administration leads to an increase in hepcidin, which causes a decrease in iron absorption in the intestine and from endogenous iron reserves. On the other hand, plasma hepcidin levels follow a circadian rhythm with an increase during the day.
Therefore, the most efficient absorption and thus the greatest possible reduction of gastrointestinal side effects results from the morning administration of the iron preparation in a dose of at least 60 mg and with an interval of at least 48 hours [15].
The tolerability of parenterally administered iron is about the same as that of enteral therapy without the frequent gastrointestinal side effects. Feared hypersensitivity reactions, including anaphylactic reactions, occur unpredictably and with Ferinject® according to the compendium with a frequency of 0.1 to 1%. Adequate monitoring of patients is therefore mandatory.
In the case of anemia in pregnancy, it is important to remember that oral iron supplementation is often insufficient in this context. National and international publications or guidelines recommend intravenous iron therapy in the second and third trimesters [16–18].
The administration of erythropoietin in the preoperative setting is recommended by the professional societies NATA, ESA, STS, and ASA with evidence 2A. The discussion about the risks and side effects of erythropoietin currently focuses on three topics:
- The increased risk of thromboembolic complications is increasingly accepted in the literature and responded to by professional societies with the recommendation for consistent TE prophylaxis [19–23].
- The data on the effect of Epo on tumor progression is not yet fully conclusive. In 2009, Bohlius demonstrated a negative impact of Epo on tumor progression in a review including more than 14 000 patients [24]. Therefore, the ESA recommends an individual risk assessment of each patient and limits its use to anemias with an Hb <110 g/l. If Hb rises to values above 120 or 130 g/l, therapy should be suspended immediately.
- The use of Epo in chronic renal failure is limited to severe anemia because of increased mortality, severe cardiovascular complications, and increased risk of stroke [25,26].
Due to these risks, which have not yet been clarified in detail, the obligation to pay for erythropoietin is currently still limited to the orthopedic setting. Only patients with
- preoperative symptomatic anemia or
- Moderate-severe anemia (Hb 100-130 g/l) and expected blood loss of 900-1800 ml, in whom hematopoietic measures are unavailable, inadequate, or contraindicated.
Expected/latent anemia
The third group of patients with normal hemoglobin with borderline ferritin of <100 μg/l, who are expected to have blood loss greater than 1000 ml or Hb drop greater than 30 g/l, should be preemptively treated with 1000 mg Fe and, for pragmatic reasons, together with 5 mg folic acid.
Time factor
This algorithm is aimed at patients prior to elective major surgery. Of course, it can only be applied successfully if the patients are also recorded preoperatively in good time and brought to a clarification. We therefore recommend initial diagnostics and appropriate therapy initiation directly after indication for surgery, ideally at least 28 days before the date of surgery.
In the absence of preparation time, preliminary data now demonstrate that parenteral iron therapy even shortly before or immediately after surgery is a pragmatic solution in which the postoperative infection rate and foreign blood transfusions are reduced and an earlier and more durable Hb rise can be observed than in the spontaneous course [27,28].
Outlook
Data on improved outcomes of patients in the elective surgical setting with respect to mortality, infections, and foreign blood transfusions have now also been confirmed in a prospective, randomized fashion [29]. After initial doubts [30], recent data now demonstrate that preoperative anemia therapy also improves outcome in the colorectal cancer patient population [31]. Correction of anemia with iron (and erythropoietin, if necessary) in the setting of heart failure and aortic stenosis also improves left ventricular pump function, decreases BNP, and reduces mortality-with and without surgery [32]. Questions about the importance of iron therapy in iron deficiency without anemia and postoperative iron supplementation will hopefully be answered soon by further studies.
The therapy of anemia without foreign blood transfusion prior to elective surgery is an integral part of modern patient blood management, is scientifically “state of the art”, improves patient safety and positively supports the healing process [33].
Acknowledgments: The authors thank Prof. Hans Gombotz for the unbureaucratic and kind provision of his presentation of the patient blood management concept and Hardowin Wohlhoff, Unilabs Horgen, for the illustrative hematology images.
Author’s Statement: The authors declare that they have no financial ties to any company whose product plays an important role in this article, nor are they related to any company that sells a competing product.
Literature:
- Fowler AJ, et al: Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg 2015; 102(11): 1314-1324.
- Shander A, et al: Iron deficiency anemia – bridging the knowledge and practice gap. Transfus Med Rev 2014; 28: 156-166.
- Musallam KM, et al: Preoperative anaemia an postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet 2011; 378 (9800): 1396-1407
- Baron DM, et al: Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 2014; 113(3): 416-423.
- Karkouti k, et al: Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008; 117(4): 478-484.
- Lasocki S, et al: PREPARE: the prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery: a multicentre, observational study. Eur J Anaesthesiol 2015; 32(3): 160-167.
- Kassebaum NJ, GBD 2013 Anemia Collaborators: The Global Burden of Anemia. Hematol Oncol Clin North Am 2016; 30(2): 247-308.
- Clevenger B, et al: Pre-operative anaemia. Anesthesia 2015; 70(Suppl 1): 20-28.
- Ng O, et al: Iron therapy for pre-operative anaemia. Cochrane Database Syst Rev 2015; 12: CD011588.
- Pattakos G, et al: Outcome of patients who refuse transfusion after cardiac surgery: a natural experiment with severe blood conservation. Arch Intern Med 2012; 172(15): 1154-1160.
- Murphy MF, et al: Transfusion practice and safety: current status and possibilities for improvement. Vox Sang 2011; 100(1): 46-59.
- Nat Kidney Foundation KDOQI: Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis 2006; 47(5 Suppl 3): 16-85.
- Gupta A, et al: Pathogenesis of anaphylactoid reactions to intravenous iron. Am J Kidney Dis 2000; 35(2): 360-361.
- Elhenawy AM, et al: Role of preoperative intravenous iron therapy to correct anemia before major surgery: study protocol for systematic review and meta-analysis. Syst Rev 2015; 4: 29.
- Moretti D, et al: Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015; 126(17): 1981-1989.
- Khalafallah AA, et al: Three-year follow-op of a randomised clinical trial of intravenous versus oral iron for anaemia in pregnancy. BMJ Open 2012; 2(5): piie000998.
- Pavord S, et al: UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2012; 2156(5): 588-600.
- Christoph P, et al: Intravenous iron treatment in pregnancy: comparison of high-dose ferric carboxymaltose vs. iron sucrose. J Perinat Med 2012; 40(5): 469-474.
- Kumar A, et al: Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med 2009; 76(Suppl 4): 112-118.
- Stowell CP, et al: An open-label, randomized, parallel-group study of perioperative epoetin alfa versus standard of care for blood conservation in major elective spinal surgery: sefety analysis. Spine 2009; 34(23): 2479-2485.
- Lippi G, et al: Thrombotic complications of erythropoiesis-stimulating agents. Semin Thromb Hemost 2010; 36(5): 537-549.
- Goodnough LT, et al: Detection, evaluation, an management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth 2011; 106(1): 13-22.
- Kozek-Langenecker SA, et al: Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol 2013; 30(6): 270-382.
- Bohlius J, et al: Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 2009; 373(9674): 1532-1542.
- Saran R, et al: Establishing a national chronic kidney disease surveillance system for the United States. Clin J Am Soc Nephrol 2010; 5(1): 152-161.
- Cody JD, et al: Recombinant human erythropoietin versus placebo or no treatment for the anemia of chronic kidney disease in people not requiring dialysis. Cochrane Database Syst Rev 2016; CD 003266.
- Khalafallah AA, et al: Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomized controlled trial. Lancet Haematol 2016; 3(9): e415-e425.
- Munoz M, et al: Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion 2014; 54(2): 289-299.
- Froessler B, et al: The Important Role for Intravenous Iron in Perioperative Patient Blood Management in Major Abdominal Surgery: A Randomized Conrolled Trial. Ann Surg 2016; 264(1): 41-46.
- Hallet J, et al: The impact of perioperative iron on the use of red blood cell transfusions in gastrointestinal surgery: a systematic review and meta-analysis. Transfus Med Rev 2014; 28(4): 205-211.
- Calleja JL, et al: Ferric carboxymaltose reduces transfusions and hospital stay in patients with colon cancer and anemia. Int J Colorectal Dis 2016; 31(3): 543-551.
- Gomez M, et al: Effect of correction of anemia on echocardiographic and clinical parameters in patients with aortic stenosis involving a three-cusp aortic valve and normal left ventricular ejection fraction. Am J Cardiol 2015; 116(2): 270-274.
- Choorapoikayil S, et al: Patient blood management: is it worth to be employed? Curr Opin Anesthesiol 2016; 29(2): 186-191.
Further reading:
- Pratt JJ, et al: Non-anaemic iron deficiency – a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol 2016; 96(6): 618-628.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(1): 10-16.