At the EHA Congress in Milan, multiple myeloma was the focus of several events. What are the current results of proteasome inhibitors and immunomodulatory drugs and what is their promise for the future? How can the therapy situation for older patients be improved? Three experts provided information about this.
(ag) Speaking on the use of pomalidomide in advanced relapsed and refractory multiple myeloma (rrMM), Prof. Meletios Dimopoulos, MD, Athens, Greece, said, “Based on currently available evidence, pomalidomide and low-dose dexamethasone is well tolerated in rrMM and is an effective treatment option for patients who have exhausted therapy with lenalidomide and bortezomib.” Optimal starting dose is 4 mg/tgl. Pomalidomide in days 1-21 of each 28-day cycle, regardless of comorbidities. According to Prof. Dimopoulos, it is not necessary to use the dosage of 4 mg/tgl. Adjust for mild to moderate renal function impairment (CrCl ≥45 ml/min). However, close monitoring of side effects must take place. Dexamethasone is administered as a 40 mg dose weekly. For patients over 75 years of age, reduce to 20 mg.
“Treatment should be continued until progression or unacceptable toxicities occur. Modification of dosage is possible in patients with neutropenia, thrombocytopenia, and other grade 3 – 4 adverse events. Prophylaxis should be considered to prevent infections and venous thromboembolism (VTE),” the expert said. One such includes, for example, administration of granulocyte colony-stimulating factor (G-CSF) in cycles 1-3 to prevent neutropenia, antibiotics (cycles 1-3) to reduce risk of infection, and thromboprophylaxis (aspirin and fractionated low-molecular-weight heparin in high-risk cases) to minimize the risk of VTE in all patients.
Therapy landscape 2014
Prof. Paul G. Richardson, MD, Boston, summarized the situation regarding new agents as follows:
Proteasome inhibitors (PI) and immunomodulatory drugs (IMiD) show significant improvements for progression-free and overall survival [1]. As their mode of action becomes better understood, new therapeutic combination options are also emerging. A serious problem is the decreasing response duration and survival rates when summing up successful salvage therapy regimens [2]. Next-generation proteasome inhibitors such as carfilzomib are currently being studied in combination with lenalidomide and dexamethasone in phase III trials.
- Immune function at baseline appears to be a key switch point for success. It may be possible to target these (with PD1/PDL1 blockade).
- Monoclonal antibodies show activity in high-risk forms of disease and represent novel pathways of action (as do other immunotherapeutics). Daratumumab, a human CD38 monoclonal antibody, demonstrated effective destruction of CD38-expressing tumor cells in in vitro studies. Clinical efficacy is therefore suspected. Currently, the synergistic effect of a combination of daratumumab, lenalidomide and dexamethasone is being investigated. The results [3] were presented at ASCO 2014 and are promising in terms of safety and activity profile.
- Many other so-called “small molecule inhibitors” offer hope. A study on the histone deacetylase (HDAC) inhibitor panobinostat was also presented by Prof. Richardson at this year’s ASCO: It showed that the combination of panobinostat plus bortezomib plus dexamethasone improved progression-free survival highly significantly (namely by a median of 3.9 months) compared to the other two agents alone. Complete response rates were also nearly doubled compared with the control arm [4].
The “age” factor
Prof. Thierry Facon, MD, Lille, spoke about treatment options for newly diagnosed MM patients who are not candidates for stem cell transplantation: “Multiple myeloma is primarily a disease of the elderly. However, older age almost always precludes transplantation.
Accordingly, what are the options for this population? In the FIRST trial [5], we showed that continuous oral therapy with lenalidomide + low-dose dexamethasone was significantly superior to the triple combination of melphalan + prednisone + thalidomide and lenalidomide + dexamethasone for 18 cycles.” Progression-free survival was 25.5, 21.2, and 20.7 months, respectively. We also found a benefit of this treatment option in the interim analysis of overall survival. So one should consider the continuous regimen as a new standard here.”
Factors associated with shorter survival during therapy with melphalan + prednisone + thalidomide and/or bortezomib include according to a metastudy [6]:
- Age (>75 years)
- Renal failure
- severe cardiac/infectious side effects
- Therapy discontinuation
A study also presented at the 2014 EHA Congress found frail patients (i.e., elderly, comorbid, cognitively and functionally in poor condition) to be at significantly increased risk for toxicities and poor outcome (overall survival) [7].
Challenges for the future
Consequently, according to Prof. Facon, great progress has been made in recent years in those MM patients who are candidates for stem cell transplantation.
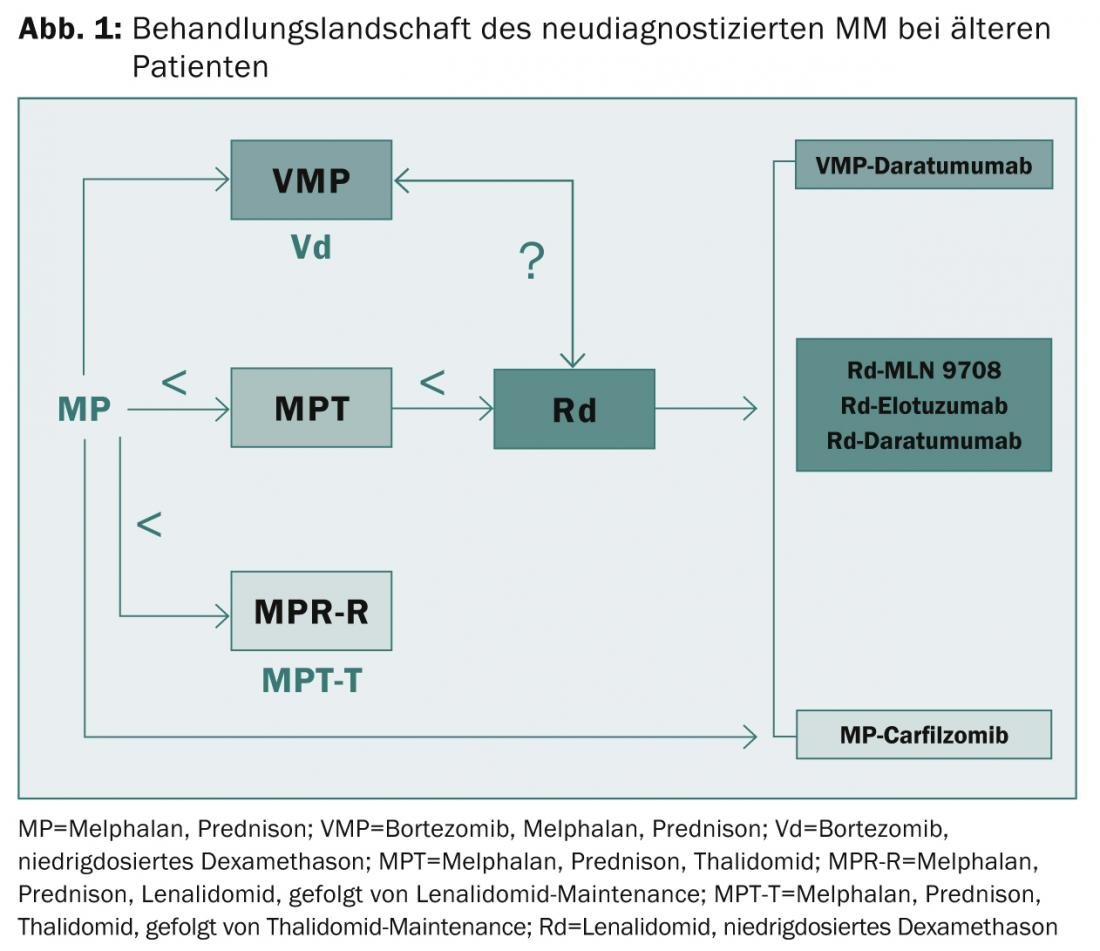
are out of the question. Median progression-free survival was extended from approximately 10-15 to 25-30 months and overall survival from approximately 30 to 60 months. “Nevertheless, high-risk patients continue to suffer from a very poor outcome even with the new first-generation agents,” the expert concluded. “Thus, the need for innovative treatment options and appropriate risk assessment remains great. Frail patients present an ongoing challenge. In this context, it may be useful to accurately assess organ function, comorbidities, frailties and disabilities,” explained Prof. Facon. “PI, IMiDs, and second- and third-generation monoclonal antibodies will play a critical role in solving these problems in the future (Fig. 1).”
Source: EHA Congress 2014, June 12-15, 2014, Milan
Literature:
- Kumar SK, et al: Blood 2008 Mar 1; 111(5): 2516-2520.
- Kumar SK, et al: Mayo Clin Proc 2004 Jul; 79(7): 867-874.
- Plesner T, et al: J Clin Oncol 2014; 32: 5s (suppl; abstr 8533).
- Richardson PG, et al: J Clin Oncol 2014; 32: 5s (suppl; abstr 8510).
- Facon T, et al: EHA 2014 #Abstract S643.
- Bringhen S, et al: Haematologica 2013 Jun; 98(6): 980-987.
- Mina R, et al: EHA 2014 #Abstract P354.
InFo Oncology & Hematology 2014; 2(6): 31-32.