As a useful plant, Lavendula angustifolia has long had traditional significance. Currently, the calming effect of the plant is experiencing a renaissance. As an orally available substance for medical use, lavender oil or silexan has become known for a few years.
Mental illness is a burden both personally for the sufferer and financially for society. Statistically, they affected about one in four Swiss in the last twelve months, accounting for about 16% of total healthcare spending in this country. Thus, mental health expenditures amount to approximately 6.1 billion Swiss francs [1]. One of the most common mental disorders is anxiety disorder [2].
In addition to the quite well-defined disorders in the group of anxiety and depressive disorders, such as generalized anxiety disorder (GAD), there are also constellations of symptoms that are more difficult to assign to a clinical picture. This includes subsyndromal anxiety disorder and mixed anxiety and depressive disorder (MADD). Both are typical symptom constellations of the full-blown clinical pictures without fulfilling all the required diagnostic criteria. Affected individuals have been shown to have the same burden as patients with full-blown syndromes, but often do not receive appropriate therapy. This is partly due to misconceptions about symptoms, but also to the wide range of side effects of orally available treatment options, such as antidepressants, neuroleptics, or tranquilizers.
Effective active ingredient from nature for anxiety disorders
Lavender or the medicinally used silexan, with its naturally relaxing and anti-anxiety properties, offers the possibility of a gentler alternative in the therapy of anxiety and depressive disorders. Lavender and the products derived from it have long been part of first the cosmetic, and later the medical industry. Silexan is produced by steam distillation from the flowers of the Lavendula angustifolia plant. As a final product, it then complies with the specifications of the European Pharmacopoeia. The medically relevant active ingredients of Silexan capsules include linalool and linalyl acetate.
In a first randomized placebo-controlled trial by Kasper et al. [3] in 2010 demonstrated that silexan is an effective treatment option with few side effects for subsyndromal anxiety disorders compared with placebo. Already with a once daily dose of 80 mg a statistically significant reduction of anxiety symptoms could be achieved. The use of lavender oil also had a positive effect on sleep quality, which is often disturbed by anxiety disorders. Sleep length and quality increased significantly. Overall, this phytotherapy subjectively improved the general mental and physical health status and thus the quality of life. These initial results were confirmed by further clinical studies. Among others, Wölk and Schläfke [4] were able to show in 2010 that medicinal lavender oil is also effective in GAD, in this case even equivalent to therapy with lorazepam. Another study on GAD by Kasper et al. [5], among others, compared the effect size of silexan and paroxetine, finding it to be 0.37 for silexan and 0.21 for paroxetine.
Mode of action
The mode of action of antianxiety and antidepressant medications is generally very complex. There are various starting points at different levels at which the respective therapeutics can exert their effect. The totality of the mode of action is not yet fully understood in the case of lavender oil. Various studies on this topic provide initial insights into the effect at the synapse level:
- In an animal study with mice, lavender oil was shown to inhibit voltage-gated calcium channels in the hippocampus and elsewhere, similar to pregabalin [6]. This subsequently leads to a reduced release of glutamate and norepinephrine, neurotransmitters that are released in increased concentrations in anxiety patients.
- A randomized, double-blind, cross-over study indicated that silexan reduced the binding potential of 5HT1A receptors, thereby increasing the extracellular concentration of serotonin [7]. The same effect is seen with SSRIs, some of which are used as first-line treatments for anxiety disorders and depression.
Efficacy also in depressive disorders
The mixed comorbidity of anxiety disorders and depression (MADD) mentioned above is particularly evident in outpatient medical care patients. Kasper et al. [8] investigated the effect of Silexan in MADD in a double-blind, randomized, placebo-controlled, multicenter study. There were 318 patients enrolled in the study and given either 80 mg of silexan or placebo 1×/d for ten weeks, with placebo containing 1/1000 of the lavender dosage of silexan for taste reasons. Endpoints were efficacy and safety of Silexan in MADD. Efficacy was measured by absolute intraindividual change on the Hamilton Anxiety Rating Scale (HAMA) and on depressive disorder-related Montgomery Asberg Depression Rating Scale (MADRS). The therapeutic effect was demonstrated by the statistically significant reduction in HAMA as well as MADRS (Fig. 1 and 2). HAMA reduced by an average of 10.8±9.6 for silexan and 8.4±8.9 points for placebo within the ten weeks of treatment (p=0.02, two-sided T-test; full analysis set; mean±SD). MADRS reduced by an average of 9.2 ± 9.9 for silexan and 6.1 ± 7.6 points for placebo, respectively (p=<0.01, two-sided T-test; full analysis set; means ± SD). Statistically significant superiority of Silexan over placebo on both scores became evident from the fourth week of the study.
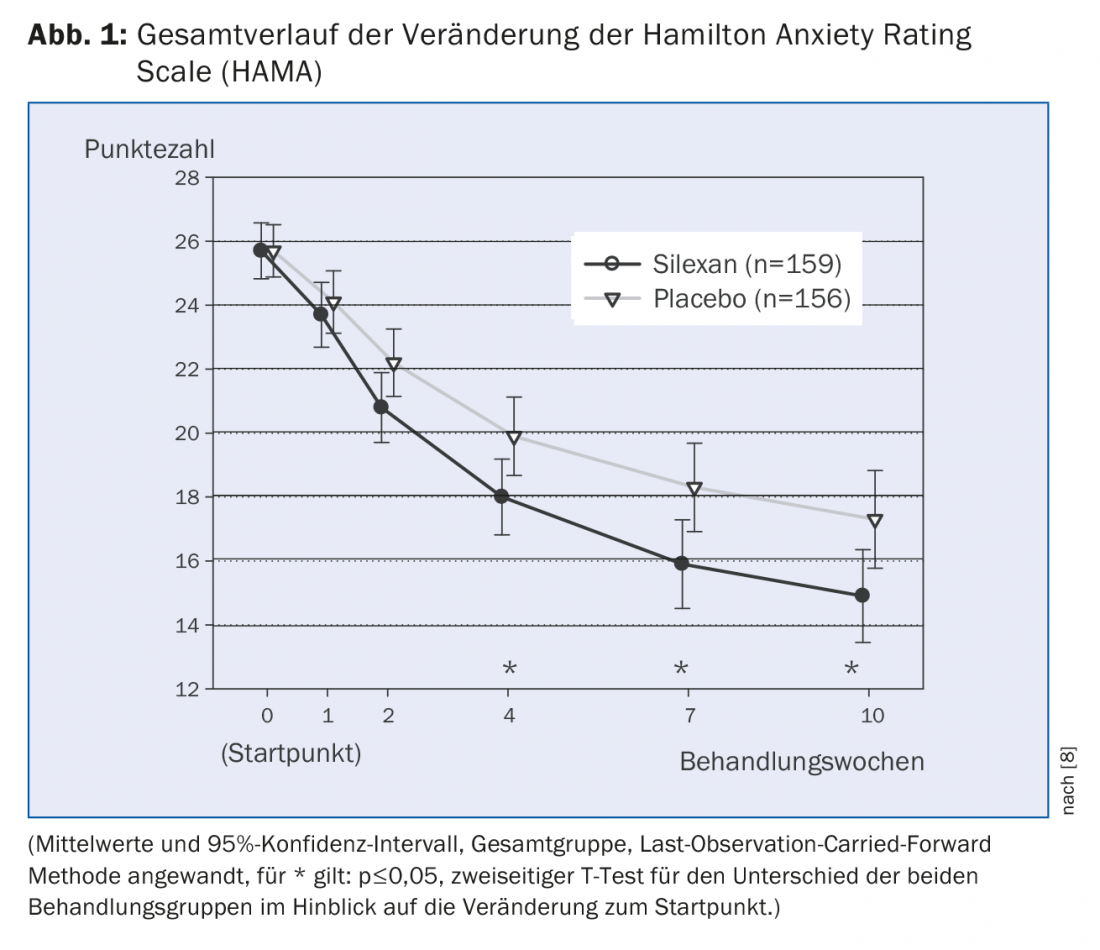
Secondary efficacy end points were a reduction of at least 50% from baseline in both scores (indicator of targeted effect) and an absolute final score of <10 for the HAMA or ≤10 points for the MADRS (indicator of remission). At least a 50% reduction was shown by 41.5% of patients for silexan and 34.6% for placebo in HAMA (p=0.21); 40.3% and 32.1%, respectively, in MADRS (p=0.13). In remission by the above definition were 34.6% for silexan and 28.8% for placebo in HAMA (p=0.27), and 46.5% and 34.0% for silexan and placebo in MADRS (p=0.02).
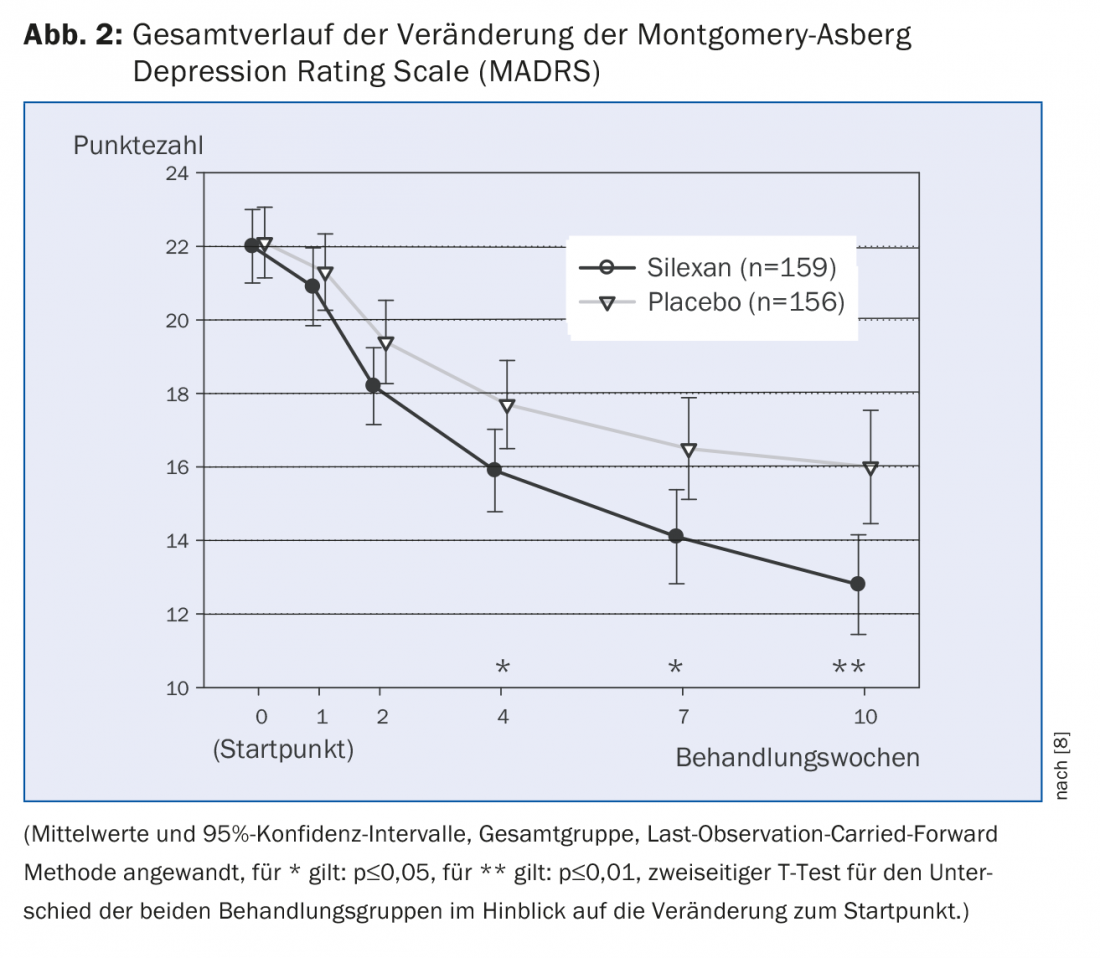
Additional scores such as the State-Trait Anxiety Inventory (STAI), the Hospital Anxiety and Depression Scale (HADS), the Clinical Global Impressions scale (CGI), the Sheehan Disability Scale (SDS), and the SF-36 health survey questionnaire were used to subjectively and objectively examine clinical outcome. Overall, the phytotherapeutic contributed to improved clinical outcome and increased health-related quality of life in patients with MADD.

Safety profile and side effect profile similar to placebo
The oral therapeutics commonly used to date all have severe side effect profiles – ranging from sedative effects to anticholinergic reactions, weight gain, headache, and sexual dysfunction. The risk of dependence symptomatology or, in the case of nonsedating antidepressants, the risk of a primary increase in the risk of suicide after initiation of therapy have also been described. All of this contributes in large part to the fact that more than half of patients with an anxiety disorder still do not receive the therapy they need [9]. In both clinical trials by Kasper et al. [3,8] Silexan was shown to have a side effect profile similar to placebo. Tolerability was investigated in both studies by means of ECG recordings, physical examinations, measurement of laboratory chemical and vital parameters, as well as the subjectively recorded side effects of the subjects. Except for possible regurgitation after ingestion of the herbal essence, no specific adverse effects have been demonstrated. In clinical practice, it is also significant that lavender oil does not interact with cytochrome P450 according to a study by Doroshyenko, et al. [10]; according to the results of another clinical study, silexan can also be given together with combined oral contraceptives [11]. It should be noted here, however, that in both of the latter analyses the study size was chosen to be quite small. Further studies in this area would be desirable, especially since the majority of patients with MADD or anxiety disorders are female.
Literature:
- Jäger M, Sobocki P, Rössler W: Cost of disorders of the brain in Switzerland with a focus on mental disorders. Swiss Med Wkly 2008 Jan; 138(1-2): 4-11.
- Hidalgo RB, Davidson JR: Generalized anxiety disorder. An important clinical concern. Med Clin North Am 2001 May; 85(3): 691-710.
- Kasper S, et al: Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of “subsyndromal” anxiety disorder: a randomized, double-blind, placebo controlled trial. Int Clin Psychopharmacol 2010; 25(5): 277-287.
- Woelk H, Schläfke S: A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine 2010 Feb; 17(2): 94-99.
- Kasper S, et al: Lavender oil preparation Silexan is effective in generalized anxiety disorder – a randomized, double-blind comparison to placebo and paroxetine. Int J Neuropsychopharmacol 2014 Jun; 17(6): 859-869.
- Schuwald AM, et al: Lavender Oil-Potent Anxiolytic Properties via Modulating Voltage Dependent Calcium Channels. PLoS One 2013 Apr; 8(4): e59998.
- Baldinger P, et al: Effects of Silexan on the serotonin-1A receptor and microstructure of the human brain: a randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. Int J Neuropsychopharmacol 2014 Oct; 18(4).
- Kasper S, et al: Efficacy of Silexan in mixed anxiety-depression–A randomized, placebo-controlled trial. Eur Neuropsychopharmacol 2016 Feb; 26(2): 331-340.
- Andrews G, Carter GL: What people say about their general practitioners’ treatment of anxiety and depression. Med J Aust 2002; 176(2): 69.
- Doroshyenko O, et al: Drug cocktail interaction study on the effect of the orally administered lavender oil preparation silexan on cytochrome P450 enzymes in healthy volunteers. Drug Metab Dispos 2013 May; 41(5): 987-993.
- Heger-Mahn D, et al: No interacting influence of lavender oil preparation silexan on oral contraception using an ethinyl estradiol/levonorgestrel combination. Drugs RD 2014 Dec; 14(4): 265-272.
HAUSARZT PRAXIS 2017; 12(6): 44-46











