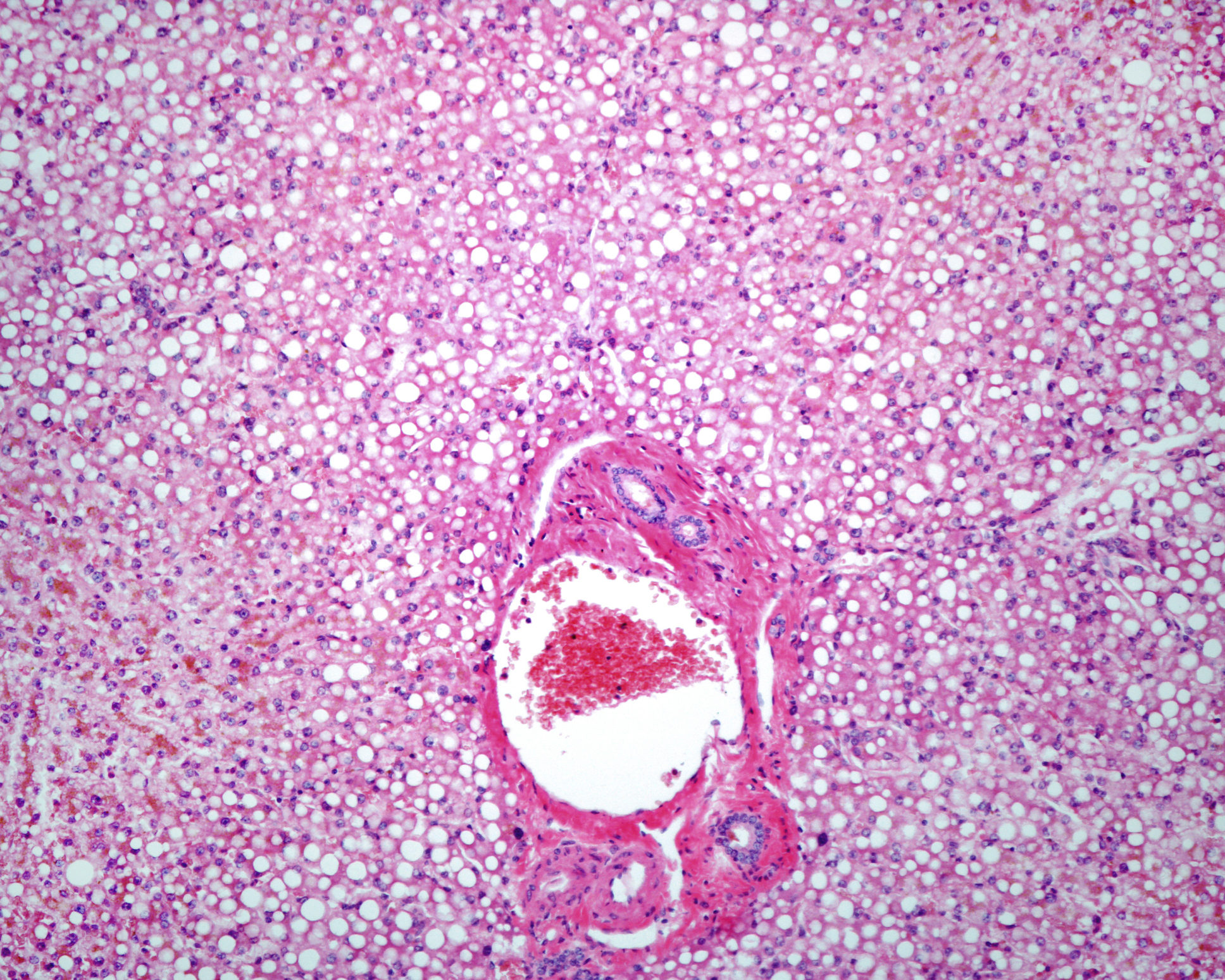
After consultation with the U.S. Food and Drug Administration (FDA), the manufacturer Biogen plans to submit aducanumab for approval. The Phase 3 EMERGE study met its primary endpoint and demonstrated a significant reduction in clinical worsening.
Aducanumab is an investigational drug that has been studied for the treatment of early Alzheimer’s disease. Patients administered the monoclonal antibody thus showed significant benefits in cognition and functional measures such as memory, orientation and language. There were also improvements in activities of daily living, including performing personal finances, household tasks, and leaving the house independently. If approved, aducanumab would be the first therapy to reduce clinical worsening of Alzheimer’s disease and the first therapy to demonstrate that amyloid beta removal leads to improved clinical outcomes.
EMERGE and ENGAGE were multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 studies evaluating the efficacy and safety of aducanumab. The primary objective of the studies was to evaluate the efficacy of monthly doses of aducanumab compared with placebo in reducing cognitive and functional impairment, as measured by changes in CDR-SB score. Secondary objective was to evaluate the effect of monthly doses of aducanumab compared with placebo on clinical decline as measured by MMSE, ADAS-Cog 13, and ADCS-ADL-MCI. In March 2019, the studies were terminated following the results of a pre-specified futility analysis based on an earlier and smaller dataset.
The decision to file for approval is based on a new analysis conducted by Biogen in consultation with the FDA. A larger data set from the studies was examined. This new evaluation includes additional data that became available only after the pre-specified futility analysis. It shows that aducanumab is pharmacologically and clinically active, as measured by dose-dependent effects in reducing brain amyloid and in reducing clinical deterioration. In both studies, the safety and tolerability profile of aducanumab was consistent with previous studies of aducanumab.
Based on discussions with the FDA, Biogen plans to submit a Biologics License Application (BLA) in early 2020. The BLA submission will include data from the Phase 1 and -1b studies as well as the full data set from the Phase 3 studies. The company intends to offer access to aducanumab to patients eligible for treatment and previously enrolled in Phase 3 trials, the long-term extension for Phase 1b PRIME trial and the EVOLVE safety trial.
Source: Biogen
InFo PAIN & GERIATURE 2019; 1(1): 39.