Interstitial lung disease (ILD) can occur as a complication of several connective tissue diseases, and increased mortality and morbidity are the consequences. ILD is thought to be driven by immune-mediated inflammation and subsequent architectural damage to the lungs. British researchers have turned their attention to treatment options for patients with systemic sclerosis, rheumatoid arthritis and inflammatory myopathies.
Pharmacotherapeutic options are mainly aimed at immunosuppression, but antifibrotic therapy has also recently shown promise. In addition, glucocorticoids are used, but they should be given only at low doses and for as short a time as possible because of the side effect profiles, write Dr. Zhe Wu and Dr. Philip Molyneaux, both of the National Heart and Lung Institute, Imperial College London, and Royal Brompton and Harefield Hospitals, London [1].
Systemic sclerosis
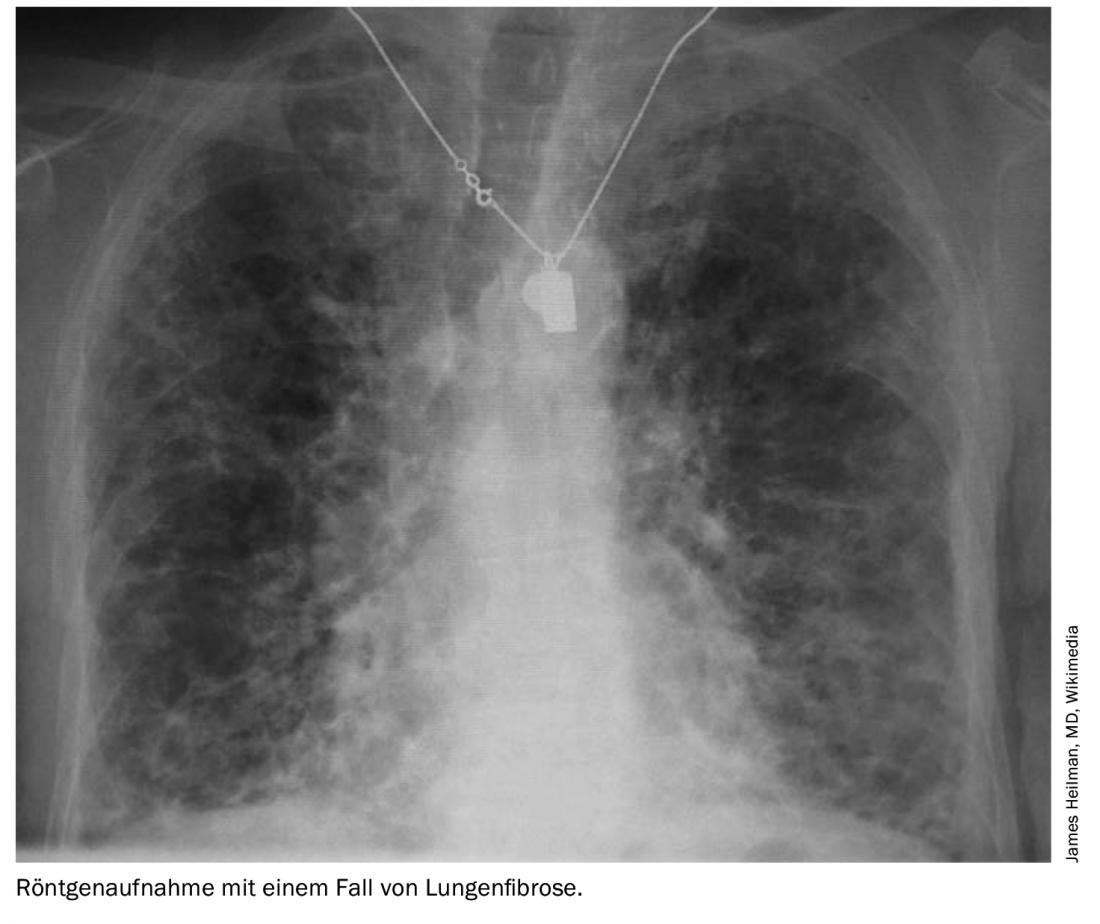
Of all connective tissue diseases, ILD occurs most frequently in systemic sclerosis (SSc) (70-90%), where it is the leading cause of death. Imaging often reveals a nonspecific pattern of interstitial pneumonia (NSIP), with a significant proportion of patients developing pulmonary hypertension.
In SSc, the extent of fibrosis determines the prognosis, and only patients with moderate to severe disease require treatment. If high-resolution computed tomography (HRCT) shows disease extension greater than 30% of the lung or involvement between 10-30% with a forced vital capacity (FVC) <70%, the physician should take therapeutic action. In the past, glucocorticoids were mostly used here. However, long-term use of steroids at doses above 10 mg prednisolone daily is associated with an increased risk of renal crisis and should be avoided.
Several randomized-controlled trials have addressed the treatment approach for steroid-sparing agents: the multicenter Scleroderma Lung Study I (SLS I) showed that oral cyclophosphamide (CYC) was effective in 1-2 mg per kgKG/day had a modest but statistically significant effect on FVC and total lung capacity (TLC) after 1 year of treatment compared with placebo (mean improvement 2.5% and 4.1%, respectively). Clinically relevant improvements were also observed with regard to dyspnea, skin changes, and functional status. In addition, CT scans of patients in the placebo arm were more likely to show progressive fibrosis. However, the FVC benefit did not persist one year after treatment cessation.
The subsequent SLS-II trial compared treatment with oral CYC for 12 months with 24 months of mycophenolate mofetil (MMF). The target daily dose of MMF was 3 g. With the same efficacy, MMF was far better tolerated, with a significantly lower risk of developing leukopenia and thrombocytopenia.
EULAR recommends CYC
The FAST trial used low-dose steroids in combination with six intravenous CYC infusions (with a dose of 600 mg/m2 per month), followed by maintenance therapy with azathioprine (2.5 mg/kgKG per day), and showed positive effects on FVC and radiological extent of disease, although these results did not reach statistical significance-possibly due to small sample size, the authors write. In addition, the cohort studied had less lung dysfunction compared with the SLS-I group. Remarkably, Dr. Wu and Dr. Molyneaux said, the side effect profile of the intravenous CYC regimen appeared to be more favorable than that of oral administration. The implications of this, he said, are important in the real-world setting, where differential compliance or even treatment discontinuation are common. As a result of these studies, the European League against Rheumatism (EULAR) recommends the use of CYC in patients with worsening lung disease.
Regarding the use of antifibrotic agents in pulmonary fibrosis, the SENSCIS trial evaluated efficacy and safety of nintedanib in SSc-ILD compared with standard therapy. The study enrolled 576 subjects with more than 10% fibrosis, about half of whom received mycophenolate mofetil at baseline. The relative reduction in FVC decline with nintedanib was 44%, similar to rates previously observed in IPF trials. However, no treatment effect on dyspnea, quality of life, or skin manifestations was observed. Nintedanib was well tolerated, with more than four-fifths of patients in the active arm completing the entire treatment period. Subsequently, post hoc analysis showed that nintedanib had similar effects on the relative reduction in FVC decline in both MMF and non-MMF groups (40% and 46%, respectively). However, the optimal timing for the introduction of antifibrotic therapy remains questionable. Smaller early phase studies with pirfenidone in SSc-ILD have now shown favorable safety and tolerability, leading to the currently ongoing SLS-III trial, which will evaluate the effect of a combination of MMF and pirfenidone compared to MMF monotherapy.
Among biologics, rituximab (RTX) has shown promise in several small trials. The multicenter RCT RECITAL compared RTX (two doses 14.5 days apart) with monthly intravenous CYC infusions (six doses) for the treatment of a range of CTDs, including SSc. The results on this are awaited. The anti-interleukin (IL)-6 agent tocilizumab has shown a potential stabilizing effect on FVC in two RCTs (faSScinate and focuSSced), although enrollment was not screened for the presence of ILD at baseline. As a result, participants had minimal pulmonary function impairment, and the efficacy of anti-IL-6 drugs in patients with moderate-to-severe ILD requires further evaluation, the authors said.
Rheumatoid arthritis
The prevalence of ILD in rheumatoid arthritis (RA) is estimated to be 5-10%, with the number increasing as screening of patients with RA increases. The predominant CT pattern is usual interstitial pneumonia (UIP), followed by NSIP, with the former giving a worse prognosis. There is general consensus that steroids should be the first-line agents, he said. The current literature does not provide conclusive evidence for steroid-sparing therapy, although RTX has shown some promising results, Wu and Molyneaux write.
The INBUILD trial evaluated the efficacy of nintedanib in multiple ILD subtypes with progressive phenotype. Although the study was not designed to specifically analyze the effect in RA patients, the overall signal was positive. Therefore, it seems likely that patients with RA and a more fibrotic UIP picture will receive antifibrotic therapy earlier in their disease course in the future.
An already historical controversy in the management of CTD-ILD is the association between methotrexate (MTX) and ILD in RA patients: Some older low-quality publications suggest that MTX is associated with fibrotic ILD, but these studies were performed before the use of CT scans. Much larger and robust studies have recently refuted the association and suggest that MTX use may delay the onset of ILD. One study contrasted MTX exposure in RA patients with ILD (n=410) versus patients without (n=673) and showed that MTX use was associated with a lower risk of developing RA-ILD (OR 0.43; 95% CI 0.26-0.69). This finding was confirmed in a multicenter prospective cohort study of over 2000 patients with newly diagnosed RA. Several other studies have further supported these findings, so MTX should no longer be routinely discontinued in rheumatoid arthritis patients with well-controlled joint disease who develop ILD, the authors conclude.
Idiopathic inflammatory myopathies.
The idiopathic inflammatory myopathies (IIMs) are a group of disorders that include polymyositis, dermatomyositis, and anti-synthetase syndrome (ASD). The most common ILD patterns are NSIP and organizing pneumonia, which can often occur simultaneously. Patients with the anti-MDA5 antibody subtype often develop a clinically amyopathic and rapidly progressive phenotype resembling acute interstitial pneumonia. They often require an aggressive and combined therapeutic approach similar to the treatment of acute interstitial pneumonia.
As with other CTD-ILDs, steroids are the first-line therapy, but relapses are common and the addition of a second-line therapeutic agent is often required to achieve remission. A systematic review concluded that intravenous CYC administration for 6-12 months improved lung function and CT appearance in more than half of patients and restored muscle strength in four-fifths. Rituximab has shown promise in a small pilot study of 10 subjects with ASD who relapsed after initial immunosuppressive therapy: In the majority of patients (9 of 10), rituximab stabilized or improved lung function. Another monocenter retrospective case series examining the long-term efficacy of RTX in 34 ASD patients (median follow-up 52 months) reported a 24% improvement in median FVC and a 17% improvement in diffusing capacity of the lung for carbon monoxide.
The calcineurin inhibitor tacrolimus has proven effective as an add-on therapy to steroids and also in combination with other immunosuppressants. However, the evidence base here is unclear, and given the high mortality, there is an urgent need to discover treatments for this cohort, the authors said. She concluded that close monitoring of lung function is generally essential to guide treatment initiation and assess response. The use of antifibrotics is a promising area, he said, but raises further questions about the timing of treatment initiation and whether it is used with concurrent immunosuppression or as a stand-alone therapy.
Literature:
- Wu Z, Molyneaux PL: Choosing pharmacotherapy for ILD in patients with connective tissue disease. Breathe 2021; 17: 210114; doi: 10.1183/20734735.0114-2021.
InFo PAIN & GERIATry 2022; 4(1-2): 26-27.