During several decades, short-acting beta-mimetics (SABA) were recommended as first-line therapy. This has changed in the meantime. The current GINA treatment recommendations advise instead the use of an anti-inflammatory demand therapy of ICS and formoterol in adults and adolescents 12 years of age and older. This recommendation is based on current scientific evidence. If conventional therapy is not effective in severe asthma, the use of biologics should be considered.
The main goals of asthma therapy include best possible symptom control and minimization of exacerbation and mortality risk, lung function loss, and medication side effects [1]. In recent years, there has been a paradigm shift with regard to therapy recommendations. This is due to the finding that SABA monotherapy does not protect even patients with mild asthma from acute exacerbations, life-threatening attacks, or death from asthma. Another factor is the sometimes considerable risk of side effects under SABA and that the inflammatory processes in the airways could not be adequately controlled. In contrast, good experience has been made with combinations of ICS and long-acting beta-sympathomimetics (long acting beta-agonist, LABA) with regard to efficacy and tolerability. Fears that use as an on-demand medication over a prolonged period of time would lead to an exacerbation of inflammatory processes in the airways were refuted in a study published in 2019 [5]. Therein it could be shown that the fixed combination budenoside/formoterol on demand did not differ from maintenance therapy with budenoside regarding the values for the inflammatory marker FeNO (fractional exhaled NO). The study period was 52 weeks.
For mild asthma, ICS and formoterol as on-demand medication.
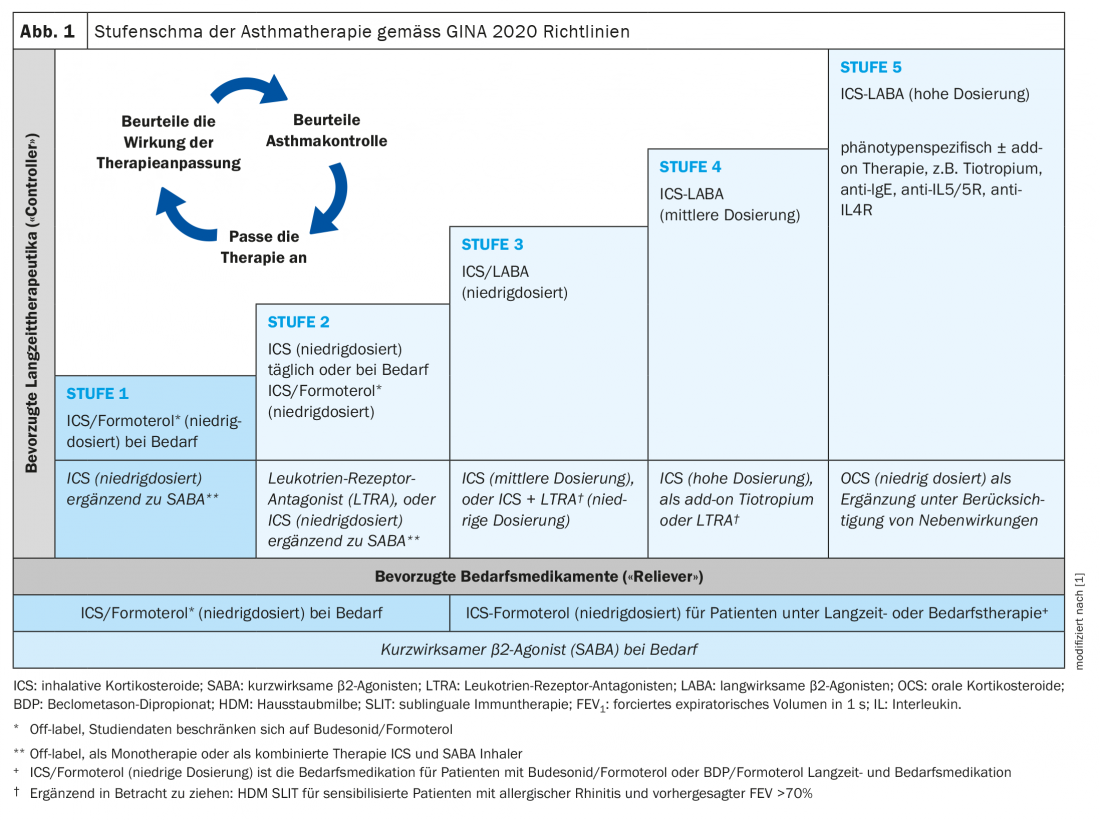
The current version of the GINA guidelines for adults and adolescents 12 years of age and older with mild asthma recommends ICS combined with formoterol as the preferred on-demand medication (Fig. 1) [1]. The criterion for mild asthma is that sufferers do not require on-demand medication more than 2× per month. In moderately severe asthma, continuous inhaled therapy with fixed combinations of ICS at varying doses and long-acting beta-mimetics is advised, supplemented with long-acting anticholinergics if necessary. Allergen immunotherapy (hyposensitization) may be considered if the allergic component of asthmatic symptoms is well documented and there is no uncontrolled asthma.
How to proceed with uncontrollable asthma?
The classification into controlled asthma, partially controlled asthma, and uncontrolled asthma is made according to the criteria presented in overview 1 [1,2]. During the course of therapy, the degree of asthma control should be checked at regular intervals (e.g. every three months) in order to be able to make therapy adjustments if necessary. What can be done about uncontrollable asthma in primary care? With reference to the current GINA guidelines, Jürg Barandun, MD, Hirslanden Lung Center, suggests the following procedure: First, inhalation technique and compliance should be checked. The next step is to check the asthma diagnosis. If spirometry reveals normal findings but the patient is symptomatic, halving the ICS dose and repeating spirometry after 2-3 weeks may be considered. Furthermore, concomitant diseases (e.g. rhinitis, depression/anxiety disorder, small airways disease) and possible influencing factors such as smoking, allergen exposure or beta blockers must be identified and eliminated. If asthma is still uncontrollable, escalation to the next level of therapy should be considered. If this does not result in control, Dr. Barandun advises further referral to pneumology specialists.
Current treatment recommendations for ICS also apply during corona pandemic. In the case of high-dose cortisone therapy, a switch to biologics may be considered [6].

In severe asthma, biologics can be targeted
To improve asthma control and lung function, and to reduce the rate of exacerbations and the need for ICS, the use of a LAMA, either in the form of a separate inhaler or in the form of triple therapy in one inhaler (ICS/LABA/LAMA), is recommended according to the GINA therapy regimen from stage 4 [3]. In stage 5, a therapeutic trial of highest-dose ICS therapy in combination with LABA and LAMA therapy may be attempted. If this does not lead to the desired symptom control or exacerbations continue to occur, it is recommended to consider the use of highly effective biologics. Long-term treatment with systemic glucocorticoids should be avoided if possible.
Biologics have been shown to reduce exacerbations and the need for corticosteroids (OCS, ICS), lead to improvement in asthma symptoms and increase quality of life. In addition to the anti IgE antibody omalizumab (Xolair®), several other options are available today for biologic therapy. These include the IL5 blockers mepolizumab (Nucala®) and reslizumab (Cinqaero®) and the IL5 receptor blocker benralizumab (Fasenra®), respectively, and the anti-IL4/13 antibody dupilumab (Dupixent®). Omalizumab can be used for severe allergic asthma, and the anti-IL5 antibody and anti-IL4/13 antibody can be used for severe eosinophilic asthma. Several empirical studies have shown that patients with severe asthma on biologics therapy are not at increased risk for severe COVID19 courses, as reported in a 2021 position statement published under the auspices of the German Society of Pneumology by Lommatzsch et al. is held [7].
Source: FomF General and Internal Medicine
Literature:
- Initiative for Asthma (GINA): Global strategy for asthma management and prevention, www.ginasthma.org
- Barandun J: Uncontrolled asthma. Jürg Barandun, MD, FOMF General and Internal Medicine, 5/15/20.
- Virchow JC, et al: Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019; 394: 1737-1749
- National Health Care Guideline (NVL) Asthma. www.leitlinien.de/nvl/asthma 2020; 4th edition, AWMF Registry No.: nvl-002.
- Beasley R, et al: Controlled Trial of Budesonide-Formoterol as Needed for Mild Asthma. N Engl J Med 2019; 380: 2020-2030.
- Vetter C: COVID-19 in patients with asthma or COPD: Inhaled steroids are not protective in COVID-19, but they do not harm either. Dtsch Arztebl 2021; 118(1-2): A-31/B-27.
- Lommatzsch M, et al: Risk assessment in patients with chronic respiratory and pulmonary diseases in the context of the SARS-CoV-2 pandemic. Statement of the DGP and BdP. Pneumology 2021; 75(01): 19-30.
- Lommatzsch M, et al: Therapy of mild and moderate asthma in adults. Dtsch Arztebl Int 2020; 117: 434-443.
Further reading:
- Guidelinesinpractice.co.uk: www.guidelinesinpractice.co.uk/respiratory/gina-asthma-strategy-whats-new-for-2020/455506.article
HAUSARZT PRAXIS 2021; 16(2): 28-29 (published 2/20-21, ahead of print).