New data confirm the results of the first Phase III study published earlier this year. In both studies, a significantly higher proportion of baricitinib-treated patients met the primary endpoint of hair growth at week 36 across both dosing regimens compared with placebo.
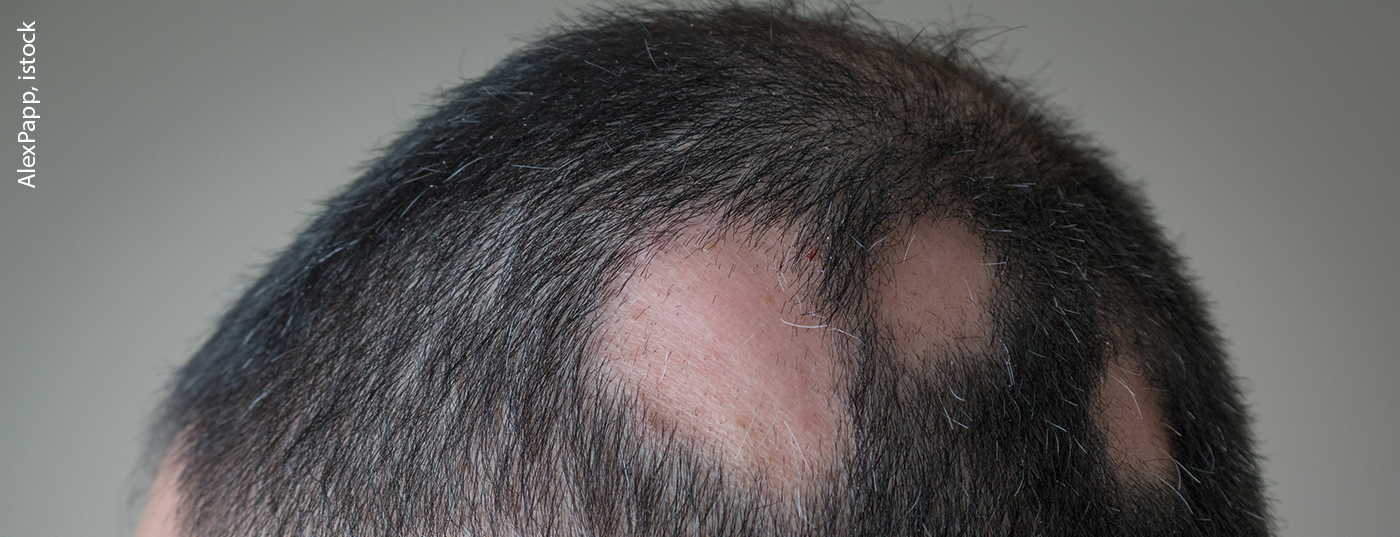
Alopecia areata (AA*) is an autoimmune disease that causes acute onset, inflammatory hair loss on the scalp, face, and sometimes other parts of the body that can progress, and for which there are currently limited treatment options. The Phase III BRAVE-AA1 study replicated the favorable efficacy and safety profile of baricitinib in adults with severe alopecia areata from the first Phase III BRAVE-AA2 clinical trial.
*AA = acute onset of hair loss due to inflammation.
Both dosages significantly superior in placebo comparison
In both studies, patients with severe alopecia areata treated with baricitinib 2 mg and 4 mg once daily over the nine-month treatment period showed significantly greater hair growth on the scalp compared to patients in the placebo group, according to physician assessment. At week 36, a significantly higher proportion of baricitinib-treated patients in the BRAVE-AA1 trial achieved 80% or greater scalp hair compared to placebo (p≤0.001). In patients treated with baricitinib 4 mg/day, this proportion was 35%; in patients treated with baricitinib 2 mg/day, it was 22%; and in the placebo group, it was 5%. In the BRAVE-AA2 trial, the proportion of patients achieving scalp hair coverage of 80% or greater was 33% of patients treated with baricitinib 4 mg/day and 17% of patients on baricitinib 2 mg/day, compared with 3% of subjects in the placebo group.
No new safety signals
The most common treatment-related adverse events in BRAVE-AA1 and BRAVE-AA2 included upper respiratory tract infections, headache, and acne. No deaths or venous thromboembolic events were reported in the studies. The safety profile of baricitinib in both studies was consistent with its known safety profile in patients with rheumatoid arthritis (RA) and atopic dermatitis (AD).
Source: “Lilly and Incyte’s Baricitinib Improved Hair Regrowth for Alopecia Areata Patients in Second Phase 3 Study,” Eli Lilly, Apr. 20, 2021.
DERMATOLOGY PRACTICE 2021; 31(3): 38