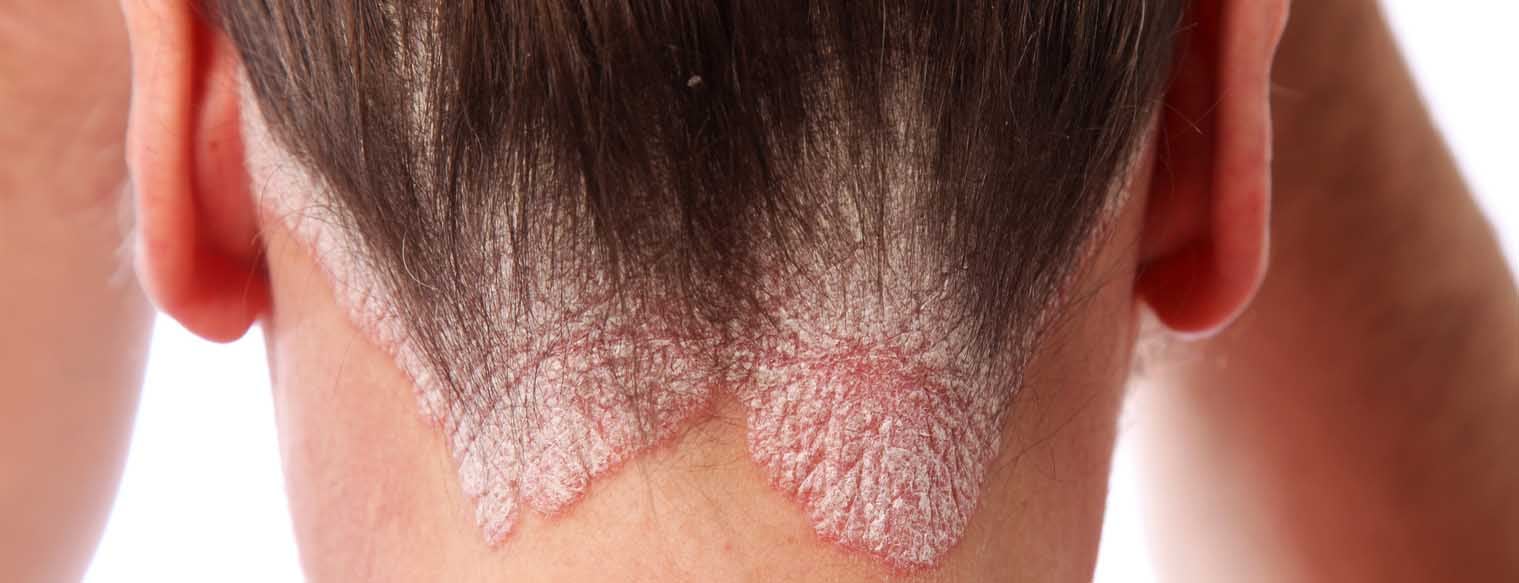
Psoriasis is a chronic inflammatory, T-cell mediated autoimmune disease that particularly affects the skin and joints. However, it is also a multisystem disease with quite a few comorbidities and has therefore become increasingly important for all medical fields, far beyond dermatology and rheumatology. The introduction of biologics brought about a profound turnaround in dermatology, benefiting not only psoriasis sufferers but also patients with other inflammatory dermatological conditions. The following article provides an overview of the pathogenesis of psoriasis and the mode of action of biologics.
Psoriasis is a chronic inflammatory disease that affects the skin and joints in particular. It affects about 2-3% of the population, making psoriasis one of the most common inflammatory skin diseases of all [1]. However, psoriasis is also a multisystemic disease with several systemic manifestations and concomitant diseases. Patients have an increased cardiovascular risk [2,3], the prevalence of metabolic syndrome is increased [4]. Depression is clustered, and psoriasis can be a pronounced psychological burden [5,6]. The risk of cancer, especially lymphoma and skin cancer, is increased [7]. In addition, psoriasis is frequently associated with other immune-mediated inflammatory diseases. For example, the incidence of Crohn’s disease is four to eight times higher than in the general population [8]. Indeed, inflammatory bowel disease, diabetes, and psoriasis have been associated with the same or similar risk genes [9,10]. Psoriasis is now considered a genetic disease with a complex interplay of genetic and external factors leading to disease development.
Psoriasis vulgaris
The classic form of psoriasis, psoriasis vulgaris, typically presents with sharply defined red plaques covered by silvery-white scales. The clinic is histologically reflected by a widened epidermis (acanthosis), elongated rete ridges (papillomatosis), a thickened stratum corneum (hyperkeratosis), and abnormal differentiation of keratinocytes with retention of nuclei in the stratum corneum (para-keratosis). This explains the clinically visible scaly plaques. The redness reflects an increased number of dilated and tortuous capillaries. The inflammatory infiltrate consists primarily of macrophages, various dendritic cells, T cells, and neutrophil granulocytes.
First therapeutic approaches
Because psoriasis is characterized by hyperkeratosis and thickened white scales caused by keratinocytes that proliferate too rapidly, it was long considered a disease of keratinocytes. It was not until the successful use of cyclosporine A and other therapeutic approaches targeting T cells that a paradigm shift occurred in the late 1970s [11,12]. Although subsequently the relevance of immune cells was no longer doubted, it remains unclear to this day which cell type is at the origin of psoriasis. Recent research results suggest that psoriasis is triggered by a combination of a primary defect in keratinocytes and an exuberant immune response [13–16].
Understanding the pathogenesis
In individuals with a genetic predisposition, various external factors such as trauma (so-called Köbner phenomenon), infections, stress or medications can trigger or later worsen psoriasis. These initial trigger factors activate the innate immune system.
Complexes of nucleic acids (DNA and RNA) and antimicrobial peptides such as LL-37 released by keratinocytes after epidermal trauma activate plasmacytoid dendritic cells and lead to the production of large amounts of interferon alpha (IFN-α) [14,15,17]. Under physiological conditions, plasmacytoid dendritic cells recognize viral nucleic acids and are essential in the induction of protective immunity [18]. In psoriasis, increased IFN-α production is critical in early disease development as it drives the autoimmune response [15]. IFN-α leads to activation and maturation of conventional dendritic cells, which in turn stimulate T cells. Thus, IFN-α links the innate to the acquired immune response. Subsequently, the autoreactive T cells proliferate and migrate into the epidermis, eventually triggering the epidermal characteristics of psoriasis such as hyperproliferation of keratinocytes and thickening of the epidermis [16].
In psoriasis, these autoreactive T cells are primarily type 1 helper T cells (Th1) and Th17 T cells, which produce interferon-gamma and interleukin (IL)-17 and IL-22, respectively [19,20]. Interestingly, the two Th17 cytokines in particular are critical in linking the acquired immune response to the epidermal dysregulation characteristic of psoriasis [21–23]. IL-22 induces hyper-proliferation of keratinocytes, and IL-17 and IL-22 both increase the production of antimicrobial peptides [24–26]. As described above, the antimicrobial peptides lead to a continuous activation of the immune system and thus, through a positive feedback loop, to the chronification of the disease. In summary, a persistently activated LL-37/IFN-α/Th17 axis is essential for psoriasis pathogenesis [18]. Indeed, several susceptibility genes provide a genetic basis for the relevance of this axis in psoriasis. Susceptibility genes such as RAGE and IFN “regulatory factor” 5 are involved in plasmacytoid dendritic cell activation and IFN-α production, respectively [27], MHC I genes are involved in autoreactive T cell activation [27,28], and IL-23 and IL-23 receptor genes are directly involved in Th17 T cell polarization and expansion [29,30].
The triumph of biologics
Over the last 15 years, this increasingly detailed understanding of pathogenesis has fundamentally changed psoriasis treatment. Based on this knowledge, a new generation of drugs known as biologics has been developed. This class of drugs is based on recombinant DNA technology and includes monoclonal antibodies and receptor fusion proteins that specifically target T cell activation or essential cytokines. Since biologics interfere very specifically with the immune response, other organs may be less affected and side effects may thus be reduced. The increasing understanding of psoriasis has opened up many new potential therapeutic approaches and, accordingly, numerous biologics are now in clinical development or are already available to physicians and patients as drugs [31].
Tumor necrosis factor (TNF) is a cytokine that plays an important role in inflammatory responses [32]. Since TNF levels are elevated in serum, skin lesions, and affected joints of psoriasis patients, TNF was an obvious candidate for initial targeted immune intervention in chronic inflammatory diseases such as Crohn’s disease, arthritis, or psoriasis. While initial studies were still based on anecdotal experience in a patient suffering from Crohn’s disease and psoriasis simultaneously [33], anti-TNF therapy later became the absolute gold standard in psoriasis treatment. Today, three different anti-TNF drugs are among the world’s ten best-selling medicines, and Humira® tops the list with sales of $10 billion.
More and more
Since the functional importance of the IFN-α/IL-23/Th17 axis in psoriasis has been recognized, a growing number of biologics targeting this axis are in clinical development. Ustekinumab, which simultaneously blocks IL-12 and IL-23 and thus inhibits the differentiation of Th1- resp. inhibits Th17 T cells, is the first drug of this group to be available on the market [34,35]. The efficacy is absolutely comparable to TNF inhibitors [36]. In contrast, briakinumab, another anti-IL-12/23 antibody, was withdrawn prior to launch despite impressive efficacy [37] due to clustered serious cardiovascular events. Although an independent meta-analysis failed to demonstrate an association between blockade of IL-12/23 and increased cardiovascular risk [38], some uncertainty remains regarding the cardiovascular safety of this group of drugs. Recently, a study demonstrated that lower serum IL-17 levels in patients with acute myocardial infarction lead to an increased risk of further cardiovascular events [39]. Accordingly, anti-IL12/23 antibodies and, in the future, IL-17 inhibitors should be used cautiously after their market introduction, at least in patients with increased cardiovascular risk. Nevertheless, the anti-IL-17 antibodies and the biologics that interfere with the IL-23/Th17 axis are likely to be among the most widely used biologics in psoriasis in the coming years, along with the already established TNF inhibitors.
A better understanding of psoriasis pathogenesis has led to a variety of new potential therapeutic approaches in recent years [31]. Although currently available biologics are effective and well tolerated, certain issues remain regarding their long-term efficacy and safety. With future advances, the availability of comprehensive genomic data from psoriasis patients, and possibly the identification of autoantigens in psoriasis, we should expect to see new breakthroughs in immunotherapy and a further growing arsenal of improved, even more targeted treatments.
Biologics outside the indication psoriasis
The success of biologics in psoriasis has led to a rethinking of dermatology as a whole and to the increasing use of pathogenesis-oriented targeted therapies in other indications. Because of the relevance of TNF in most chronic inflammatory responses, TNF inhibitors were tested in other indications early after their market introduction. Today, they are used successfully for numerous inflammatory dermatoses: TNF blockade, for example, shows very good efficacy in therapy-resistant pyoderma gangraenosum [40] and has even found its way into the guidelines for the treatment of hidradenitis suppurativa. Today, anti-IgE antibodies are used in chronic urticaria [41], and IL-1 blockade is standard therapy of autoinflammatory syndromes [42]. In addition, based on the fundamental understanding of the diseases and the immune response, biologics are increasingly being developed for specific dermatological diseases. Thus, targeted immunotherapy with antibodies against CTLA-4 and PD-1/PD-L1 in melanoma has ushered in a new era of antitumor therapy. The treatment is based on the blockade of molecules that negatively regulate an effective antitumor T-cell response (so-called T-cell checkpoint inhibition). High response rates and sustained remissions can now be achieved in melanoma, particularly with combination strategies [43].
CONCLUSION FOR PRACTICE
- The increasingly detailed understanding of disease pathogenesis has fundamentally changed the treatment of psoriasis in the last decade and led to the introduction of biologics in dermatology.
- Today, psoriasis is considered a model disease for other chronic inflammatory diseases and is frequently used in so-called “proof-of-concept” studies to investigate new therapeutic approaches that specifically intervene in the pathogenesis.
- The success of biologics in psoriasis caused a rethink in dermatology as a whole. The use of pathogenesis-oriented targeted therapies in other indications is increasing.
Literature:
- Christophers E: Psoriasis – epidemiology and clinical spectrum. Clin Exp Dermatol 2001; 26(4): 314-320.
- Mehta NN, et al: Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 2010; 31(8): 1000-1006.
- Gelfand JM, et al: Risk of myocardial infarction in patients with psoriasis. JAMA 2006; 296(14): 1735-1741.
- Gisondi P, et al: Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol 2007; 157(1): 68-73.
- Kurd SK, et al: The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol 2010; 146(8): 891-895.
- Krueger G, et al: The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol 2001; 137(3): 280-284.
- Gelfand JM, et al: The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006; 126(10): 2194-2201.
- Najarian DJ, Gottlieb AB: Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol 2003; 48(6): 805-821.
- Wolf N, et al: Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J Med Genet 2008; 45(2): 114-116.
- Nair RP, et al: Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome-wide scan. Hum Mol Genet 1997; 6(8): 1349-1356.
- Mueller W, Herrmann B: Cyclosporin A for psoriasis. N Engl J Med 1979; 301(10): 555.
- Gottlieb SL, et al: Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med 1995; 1(5): 442-447.
- Sano S, et al: Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 2005; 11(1): 43-49.
- Lande R, et al: Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449(7162): 564-569.
- Nestle FO, et al: Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 2005; 202(1): 135-143.
- Conrad C, et al: Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 2007; 13(7): 836-842.
- Ganguly D, et al: Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 2009 Aug 31; 206(9): 1983-1994.
- Conrad C, Meller S, Gilliet M: Plasmacytoid dendritic cells in the skin: to sense or not to sense nucleic acids. Semin Immunol 2009; 21(3): 101-109.
- Uyemura K, et al: The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol 1993; 101(5): 701-705.
- Lowes MA, et al: Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008; 128(5): 1207-1211.
- Tonel G, Conrad C: Interplay between keratinocytes and immune cells–recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol 2009; 41(5): 963-968.
- Zheng Y, et al: Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007; 445(7128): 648-651.
- Tonel G, et al: Cutting edge: A critical functional role for IL-23 in psoriasis. J Immunol 2010; 185(10): 5688-5691.
- Liang SC, et al: Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203(10): 2271-2279.
- Wolk K, et al: IL-22 increases the innate immunity of tissues. Immunity 2004; 21(2): 241-254.
- Peric M, et al: IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol 2008; 181(12): 8504-8512.
- Sánchez FO, et al: IFN-regulatory factor 5 gene variants interact with the class I MHC locus in the Swedish psoriasis population. J Invest Dermatol 2008; 128(7): 1704-1709.
- Nair RP, et al: Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet 2006; 78(5): 827-851.
- Cargill M, et al: A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 2007; 80(2): 273-290.
- Capon F, et al: Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet 2007; 122(2): 201-206.
- Flatz L, Conrad C: Role of T-cell-mediated inflammation in psoriasis: pathogenesis and targeted therapy. Psoriasis: Targets and Therapy 2013; 3: 1-10.
- Baugh JA, Bucala R: Mechanisms for modulating TNF alpha in immune and inflammatory disease. Curr Opin Drug Discov Devel 2001; 4(5): 635-650.
- Oh CJ, Das KM, Gottlieb AB: Treatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol 2000; 42(5 Pt 1): 829-830.
- Leonardi CL, et al. (PHOENIX 1 study investigators): PHOENIX Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008; 371(9625): 1665-1674.
- Papp KA, et al. (PHOENIX 2 study investigators): Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008; 371(9625): 1675-1684.
- Griffiths CE, et al. (ACCEPT Study Group): Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362(2): 118-128.
- Reich K, et al: A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med 2011; 365(17): 1586-1596.
- Ryan C, et al: Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA 2011; 306(8): 864-871.
- Simon T, et al: Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur Heart J 2013 Feb; 34(8): 570-577.
- Conrad C, Trueb RM: Pyoderma gangrenosum. J Dtsch Dermatol Ges 2005; 3(5): 334-342.
- Maurer M, et al: Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013 Mar 7; 368(10): 924-935.
- Caorsi R, Federici S, Gattorno M: Biologic drugs in autoinflammatory syndromes. Autoimmune Rev 2012 Nov; 12(1): 81-86.
- Ott PA, Hodi FS, Robert C: CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013 Oct 1; 19(19): 5300-5309.
DERMATOLOGIE PRAXIS 2014; 24(5): 6-8