In tumor patients, treatable causes of anemia should also be deliberately sought before transfusion. No transfusion due to falling below a “transfusion trigger”: The indication for a transfusion should always be made in conjunction with the clinical situation. A restrictive transfusion strategy is also appropriate in tumor patients. In hemato-oncologic patients, irradiated blood products may be indicated. Erythropoiesis-stimulating agents (erythropoietin, darbopoietin) are an alternative to transfusions in individual cases, but only in cases of anemia under chemotherapy. In the curative situation, they should be avoided.
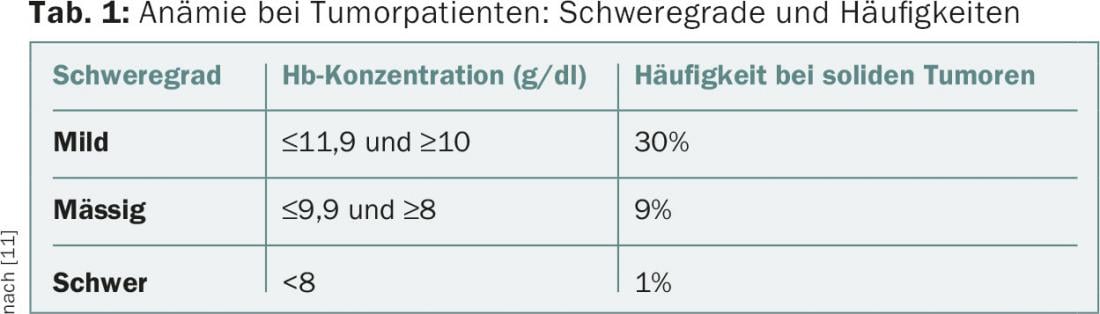
Anemia is a common problem in tumor patients (Table 1) . It affects quality of life, is an important cofactor of tumor-associated fatigue, and is a negative prognostic factor in many tumor entities. The etiology of anemia is usually multifactorial. In addition to comorbidities (e.g., renal insufficiency, deficiency states), tumor disease (hemorrhage, hypersplenism, bone marrow infiltration, proinflammatory cytokine milieu) and its treatment (myelosuppressive and nephrotoxic effect of chemotherapy and/or radiotherapy, rarely drug-induced hemolysis) contribute to its development.
Treatment is preferably causal, often only supportive measures are possible. Blood transfusions and the administration of erythropoiesis-stimulating agents (ESA) are available for this purpose. Some aspects of blood transfusion in tumor patients are highlighted below. The guidelines valid in Switzerland for pretransfusion assessment as well as selected aspects of practical implementation have recently been presented in the Swiss Medical Forum and are not mentioned here separately [1].
Clarifications before transfusion
Baseline assessments before starting transfusion should ensure that treatable other causes of anemia are not overlooked. Assessments should be made prior to initiation of transfusions and in the event of an unforeseen course, such as an increase in transfusion frequency.
Visual assessment of the blood smear provides evidence of a contributory cause of the anemia, such as dysplasia signs in myelodysplastic syndrome (MDS), microspherocytes in autoimmune hemolysis, or fragmentocytes in thrombotic microangiopathy. The determination of reticulocytes allows to distinguish hypo- from hyperregenerative anemias, whereas a hyperregenerative anemia is always an indication of increased consumption with intact synthesis capacity – a situation that is unusual in the context of tumor anemia and requires further clarification.
Substrate deficiency search includes iron status (iron, transferrin, transferrin saturation, ferritin and CRP, soluble transferrin receptor in case of unclear findings), vitamin B12 (holotranscobalamin in case of borderline findings, possibly methylmalonic acid and homocysteine) and erythrocytic folic acid. Creatinine determination reveals concomitant renal dysfunction; in manifest renal dysfunction, determination of erythropoietin is useful. Increased LDH and decreased haptoglobin indicate hemolysis. A Coombs test is primarily indicated in patients with chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, or a history of autoimmune disease.
If a longer transfusion dependency is foreseeable (e.g. in case of MDS or long therapy duration), it may be useful to characterize the antigen pattern of the patient’s erythrocytes with regard to other blood group systems in addition to the obligatory pre-transfusion clarifications in order to be able to select erythrocyte concentrates more specifically. This can reduce the likelihood of alloantibody formation, which complicates further care. Once the first transfusion has been performed, these serological tests are no longer possible and molecular biological methods must be used.
Transfusion trigger and transfusion dose
The goal of transfusion is to minimize anemia-related symptoms and prevent hypoxia-related organ damage. The potential benefit must be contrasted with transfusion-associated side effects (Table 2) [2].

The indication cannot be made dependent on falling below a “transfusion trigger” alone, since the clinical consequences depend not only on the extent of anemia, but also on the type of occurrence, duration, and clinical context of the anemia. Severe symptoms are more likely with rapid onset, whereas multilayered compensatory mechanisms (e.g., increased cardiac output, adaptation of oxygen binding capacity, altered oxygen consumption, and adapted oxygen extraction in target tissues) kick in when anemia develops slowly [3]. Pre-existing pulmonary and cardio- or cerebrovascular disease often limits adaptive capacity. The indication for transfusion thus depends crucially on the patient situation and is always an individual clinical decision (Tab. 3).

In general, a restrictive transfusion strategy (transfusion when Hb is ≤7-9 g/dl) can be distinguished from a liberal one (transfusion when Hb is ≤9-10 g/dl). Evidence from randomized trials shows that a restrictive strategy does not provide quo ad vitam disadvantages but helps to avoid transfusion-associated complications and reduce costs. According to a Cochrane analysis, a restrictive transfusion strategy leads to a statistically significant reduction in hospital-associated mortality and does not increase the rate of adverse events such as myocardial infarction or cerebrovascular insults [4]. However, the prospective data are mostly from the context of perioperative anemia management or critical care and do not relate to outpatients. No randomized prospective studies exist for tumor patients, but a restrictive transfusion strategy is also generally accepted for this patient group. Table 4 shows the recommendations for transfusion in tumor patients given in the current NCCN guidelines (February 2015) [5].
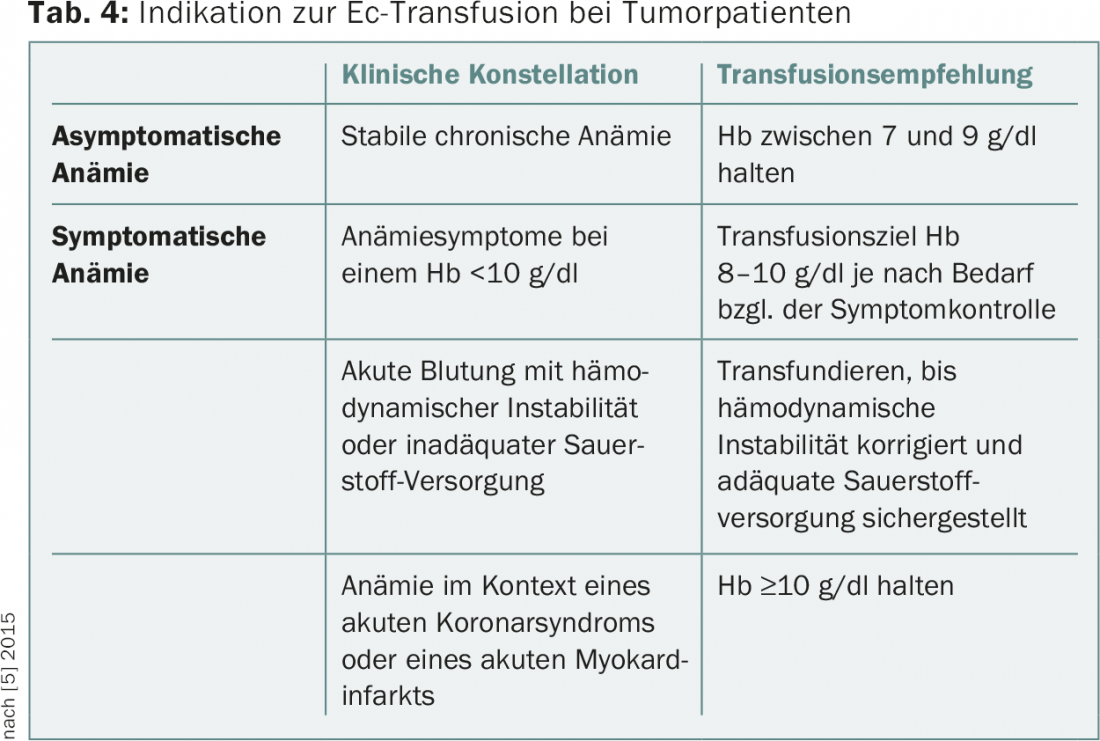
For patients with concomitant severe thrombocytopenia, it should be noted that the risk of bleeding increases with decreasing hemoglobin concentration. Therefore, it is recommended to maintain hemoglobin >8 g/dl in thrombopenic patients.
An erythrocyte concentrate (EC, volume approx. 300 ml) leads to an increase in Hb concentration of approx. 1 g/dl. Following an unwritten dogma, two ECs are usually administered per transfusion. However, there is evidence that transfusion of a single EC may also be effective, at least in hospitalized patients undergoing intensive chemotherapy [6]. In the context of an individual outpatient transfusion program, this is also an option, for example in patients with cardiac comorbidity or if major hemoglobin fluctuations are poorly tolerated with a longer transfusion interval.
If subjective symptoms play a role in the indication, it is advisable to record them semi-quantitatively before the first transfusion and to document their course during therapy (for example, using a visual analog scale).
Irradiated blood products in patients with immunosuppression
Patients with severe therapy-related immunosuppression are at risk of transfusion-associated graft-versus-host disease (ta-GvHD). In this case, donor T lymphocytes contained in the blood product are directed against recipient tissue because they cannot be recognized as foreign and eliminated. the disease is fatal in >90% of cases. After the introduction of leukocyte depletion (which limits the leukocyte content of a blood product to <1× 106), the already very low index rate has further decreased, but isolated cases have been reported even after administration of leukocyte-depleted blood products [7].
After irradiation (min. 25 Gy), lymphocytes in the blood product are no longer able to divide and cannot cause ta-GvHD. The indications for the administration of irradiated blood products according to current guidelines are summarized in Table 5 [8,9]. No binding guidelines exist for Switzerland. In addition to indisputable indications (patients after hematopoietic stem cell transplantation, therapy with purine analogues), there are situations that are evaluated differently.
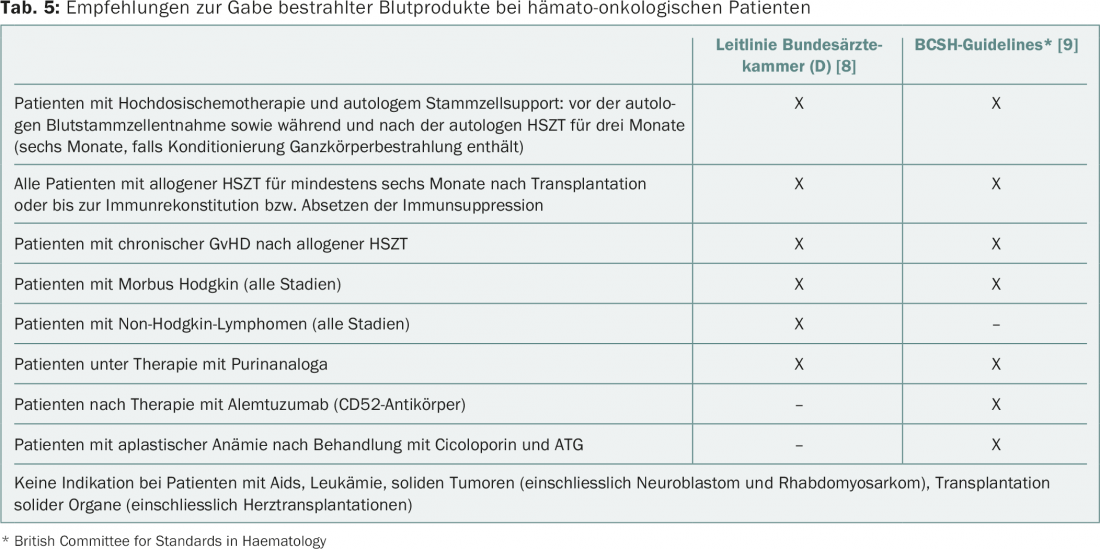
The pathogen inactivation of platelet concentrates, which is practiced throughout Switzerland, has the same effect as irradiation. Therefore, only EC and granulocyte concentrates, which are in any case only used in very special indications, need to be irradiated.
Iron chelation in long-term transfusion dependence.
Each EK contains about 200-250 mg of iron. Since the body can only eliminate iron through blood loss, clinically relevant iron overload can be expected from about 20 EC. Long-term regular Ec substitution thus leads to fatal cardiac or hepatic side effects. Therefore, iron chelation is standard in benign hematologic disorders such as thalassemias.
It is unclear whether this can also improve prognosis in long-term transfusion-dependent MDS patients. Current guidelines mention the option of iron chelation mainly in transfusion-dependent patients with low- or intermediate-risk MDS, a ferritin concentration of >1000 mcg/l, and a life expectancy of >1-2 years [10].
Erythropoiesis-stimulating agents as an alternative to transfusion
Recombinant erythropoietin and darbopoietin are part of the standard repertoire in patients with low-risk MDS and inadequately increased endogenous EPO production; these agents have been intensively promoted for use in tumor patients as well. In selected patients, they can reduce transfusion frequency and improve quality of life. However, there is evidence that in solid tumors, stimulation of e.g. erythropoietin receptors on tumor cells as well as off-target effects may have an unfavorable impact on the course of the disease, although the data in this regard are complex and controversial. In addition, erythropoiesis-stimulating agents (ESAs) increase the risk of thromboembolic events.
According to the European Medical Agency (EMA) label, their use is indicated only in chemotherapy-associated anemia starting at a Hb ≤10 g/dL; the goal is to keep the hemoglobin concentration stable or to raise it by a maximum of 2 g/dL. When administered with a target Hb >12 g/dL, increased mortality is indicated. In the adjuvant setting, ESAs should be used with caution [11].
I would like to thank my colleagues Dr. Christina Appenzeller and Prof. Dr. Christoph Driessen for their critical review of the manuscript.
Literature:
- Fontana S, Rigamonti V: Transfusion of blood products. Switzerland Med Forum 2013; 13(05): 89-93.
- Fopp M, Wernli M: Safety of blood transfusion today. Switzerland Med Forum 2006; 6: 139-144.
- Klein HG, et al: Red blood cell transfusion in clinical practice. Lancet 2007; 370 (9585): 415-426.
- Carson JL, et al: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2012; 4: CD002042.
- National Clinical Practice Guidelines in Oncology: Cancer- and chemotherapy-induced anemia 2.2.01. www.nccn.org/professionals/physician_gls/pdf/anemia.pdf (accessed 02/27/15)
- Berger MD, et al: Significant reduction of red blood cell transfusion requirements by changing from a double-unit to a single-unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica 2012; 97(1): 116-122.
- Williamson LM, et al: The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion-associated graft-versus-host disease. Transfusion 2007; 47(8): 1455-1467.
- Bundesärztekammer: Querschnitts-Leitlinien (BÄK) zur Therapie mit Blutkomponenten und Plasmaderivaten – 4. aktualisierte und überarbeitete Auflage, 2014. www.bundesaerztekammer.de/downloads/QLL_Haemotherapie_2014.pdf (accessed 27.02.2015)
- Treleaven J, et al: Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. British Journal of Haematology 2010; 152: 35-51.
- Malcovati L, et al: Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European Leukemia Net. Blood 2013; 122(17): 2943-2964.
- Schrijvers D, et al: Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinica Practice Guidelines for use. Ann Oncol 2010; 21 Suppl 5: v244-247.
- Salama A, Welte M: Therapy with erythrocytes. In: Transfusion Medicine and Immunohematology, Berlin Heidelberg New York 2010, 311-319.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(3-4): 15-18.