At the 26th Physicians’ Continuing Education Course in Clinical Oncology in St. Gallen, Symposium 1 focused on various therapeutic options for breast cancer. In early, estrogen receptor-positive breast carcinoma, can the additional administration of bone resorption-inhibiting therapy improve survival? And what treatment options are available when endocrine therapy fails in a patient with metastatic breast cancer?
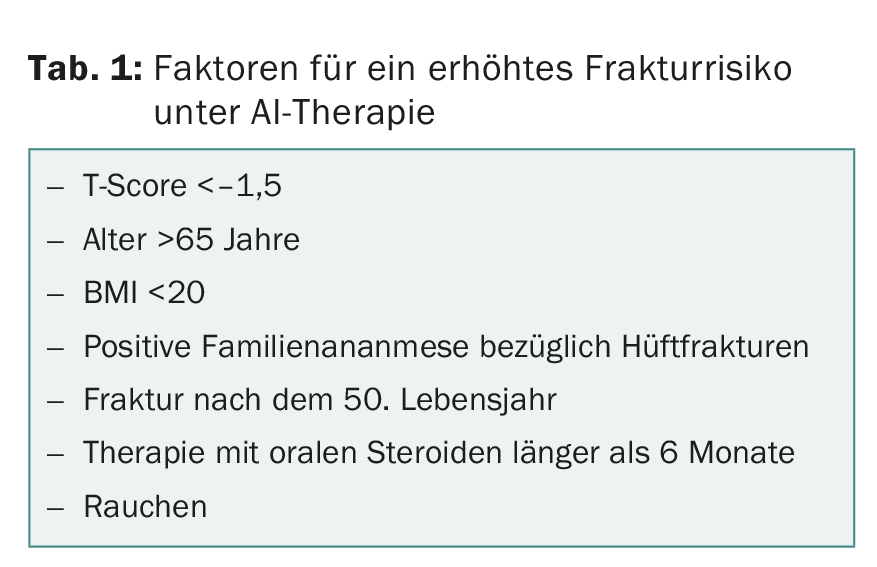
All aromatase inhibitors (AIs) used to treat breast carcinoma increase the risk of fractures. For this reason, the administration of vitamin D and calcium is now recommended for all women on AI therapy, and the administration of a bisphosphonate or RANKL inhibitor is recommended for women with an additional increased risk of fracture (two or more risk factors, Table 1). Bone density should be measured before starting AI treatment and every two years thereafter.
PD Dr. med. Roger von Moos, Kantonsspital Graubünden, Chur, provided information on the current evidence of the impact of BMA (bone modifying agents) on progression-free survival (PFS) and overall survival (OS).
Inhibition of bone resorption: postmenopausal patients benefit
The relationship between BMA and PFS resp. OS has been investigated in the context of various studies. In the largest, the AZURE trial, 3360 breast cancer patients (stages II/III, estrogen receptor-positive) received either standard therapy or additional treatment with zoledronate [1]. Follow-up lasted up to seven years. During this time, there was no significant difference in disease-free survival and OS between the two treatment groups. However, subgroup analyses showed up to 30% improved OS in patients who were over 60 years of age or more than five years postmenopausal. “However, survival in breast cancer patients in this high-risk group is very high anyway, at over 95% at eight years,” the speaker noted. “So it’s very difficult to prove the beneficial effect of an additional treatment option.”
In the ZO-FAST study, which was also conducted with zoledronate [2], and in the B-34 study, in which patients took clodronate [3], there was a slight advantage for the verum group in each case – again mainly in postmenopausal women. A recent meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) published in the Lancet in October 2015 concluded that adjuvant treatment with bisphosphonates improves survival, but only in postmenopausal women. The reduction in breast cancer-specific mortality in this patient group after ten years is approximately 3.3% [4].
A planned interim analysis of the ABCSG-18 trial presented at the 2015 San Antonio Congress showed an improvement in disease-free survival (HR of 0.816) for adjuvant administration of denosumab every six months. Patients with T2/3 and ER/PGR-positive tumors benefited most. Even with denosumab therapy, however, there are still many unanswered questions, such as at what frequency and for how long the drug should be given. Answers are hoped for from the D-CARE study, the results of which are expected at the end of 2016.
Dr. von Moos’ preliminary conclusion: “Adjuvant therapy with a BMA should be discussed with each patient individually – both in terms of bone health and disease-free survival.”
Switching from hormone to chemotherapy in metastatic breast cancer.
When to switch from hormonal to chemotherapy in patients with metastatic, HR-positive, HER2-negative breast cancer? Prof. Dr. med. Stefan Aebi, Cantonal Hospital Lucerne, spoke on this question. Primarily, these patients are treated with endocrine-disrupting drugs. First-line therapy often works for about a year – but the variability is very high – and follow-up therapies for a shorter time. With second-line or even third-line endocrine therapies, partial remission or a so-called clinical benefit (no progression of the disease for at least 24 weeks) can be achieved in some patients. Drug dosage may play a role in treatment response, as shown in the CONFIRM study: Clinical benefit was higher under treatment with 500 mg than under 250 mg (46% vs. 40%) [5]. The sequence of the different drug groups (AI, SERM, SERD) is irrelevant for the therapeutic benefit.
The addition of targeted therapies such as everolimus or bevacizumab can increase treatment response and PFS, but has not yet been shown to increase OS. “The patient’s quality of life and costs must also be considered,” the speaker indicated. Significantly more grade 3 and 4 adverse effects occurred with exemestane + everolimus treatment compared with exemestane + placebo (stomatitis: 8% vs. 1%, anemia: 6% vs. <1%, dyspnea: 4% vs. 1%, etc.) [6]. In addition, treatment with everolimus costs around 4600 Swiss francs per month – in Switzerland, this therapy is paid for by health insurers, but not in the UK.
Switching from endocrine treatment to palliative chemotherapy should be considered when endocrine therapy can no longer halt disease progression and/or a rapidly effective measure is required because of high symptom burden (“visceral crisis,” severe organ dysfunction due to progression). Guidelines on when and how to make this decision do not exist – it depends on the patient’s wishes and the course of the disease.
Source: 26th Physician Continuing Education Course in Kinic Oncology, February 18-20, 2016, St. Gallen.
Literature:
- Coleman R, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011; 365: 1396-1405.
- Eidtmann H, et al: Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann 1Oncol 2010; 21(11): 2188-2194.
- Paterson AH, et al: Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34). A multicentre, placebo-controlled, randomised trial. Lancet Oncol 2012; 13: 734-742.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Adjuvant bisphosphonate treatment in early breast cancer. Meta-analyses of individual patient data from randomised trials. Lancet 2015 Oct 3; 386(10001): 1353-1361.
- Di Leo A, et al: Final overall survival. Fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2014; 106(1):djt337.
- Baselga J, et al: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366(6): 520-529.
- Hadji P, et al: Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer. Practical guidance for prevention and treatment. Ann Oncol 2011; 22: 2546-2555.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(2): 43-44.











