Studies such as CESAR or SHOCK II, but also the further development of pumps, oxygenators and perfusion tubing have contributed to the fact that therapy with ECMO and ECLS has become much more important. These procedures are challenging for the interdisciplinary treatment team.
ECMO stands for “extracorporeal membrane oxygenation” and refers to an extracorporeal centrifugal pump with an oxygenator that is connected to the patient’s circulation via a tube system. A distinction is made between veno-venous ECMO, which is used in acute respiratory failure, and veno-arterial ECMO, which is used in patients in acute cardiogenic shock. Recently, the nomenclature has been specified into the designation ECMO for the veno-venous (vv) configuration and ECLS (“Extracorporeal Life Support”) for the veno-arterial (va) configuration.
Therapy with ECMO/ECLS has rapidly gained importance in recent years. This was initially due to the results published in the CESAR trial, which showed that patients with acute respiratory distress syndrome (ARDS) treated with veno-venous ECMO had an advantage in terms of survival and quality of life compared to patients receiving ARDS-targeted ventilation therapy alone [1]. Implantation of vaECLS in cardiogenic shock has also experienced an extraordinary upsurge. On the one hand, this was due to technical advances in pumps, oxygenators and perfusion tubing. On the other hand, the SHOCK II trial, which failed to show a survival benefit in cardiogenic shock with the use of intra-aortic balloon pump (IABP) [2], paved the way for the use of vaECLS.
The management of patients at vvECMO or vaECLS is particularly challenging. Implantation of the system and subsequent treatment of the patient requires an interdisciplinary, multi-professional team, including intensivists, cardiac surgeons, cardiologists and pulmonologists, as well as perfusionists and critical care nurses.
Cannulation and configuration of vvECMO and vaECLS
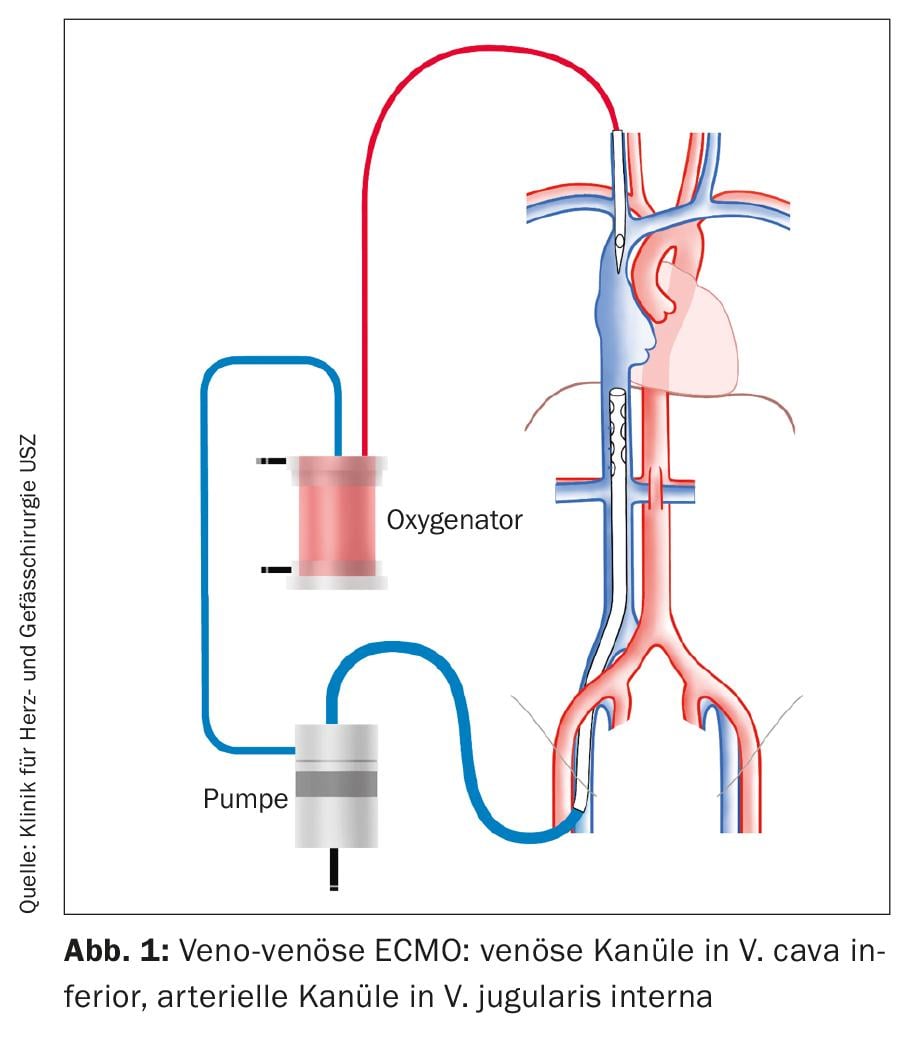
As a procedure for acute respiratory failure, ECMO is implanted in a veno-venous configuration (Fig. 1). For venous drainage, a cannula is inserted into the common femoral vein (VFC). The tip of the cannula is placed under echocardiographic control so that it lies at the entrance of the inferior vena cava (VCI) into the right atrium. A cannula is inserted into the internal jugular vein (VJI) to deliver the oxygenated blood. The tip of the cannula is located in the superior vena cava (VCS).
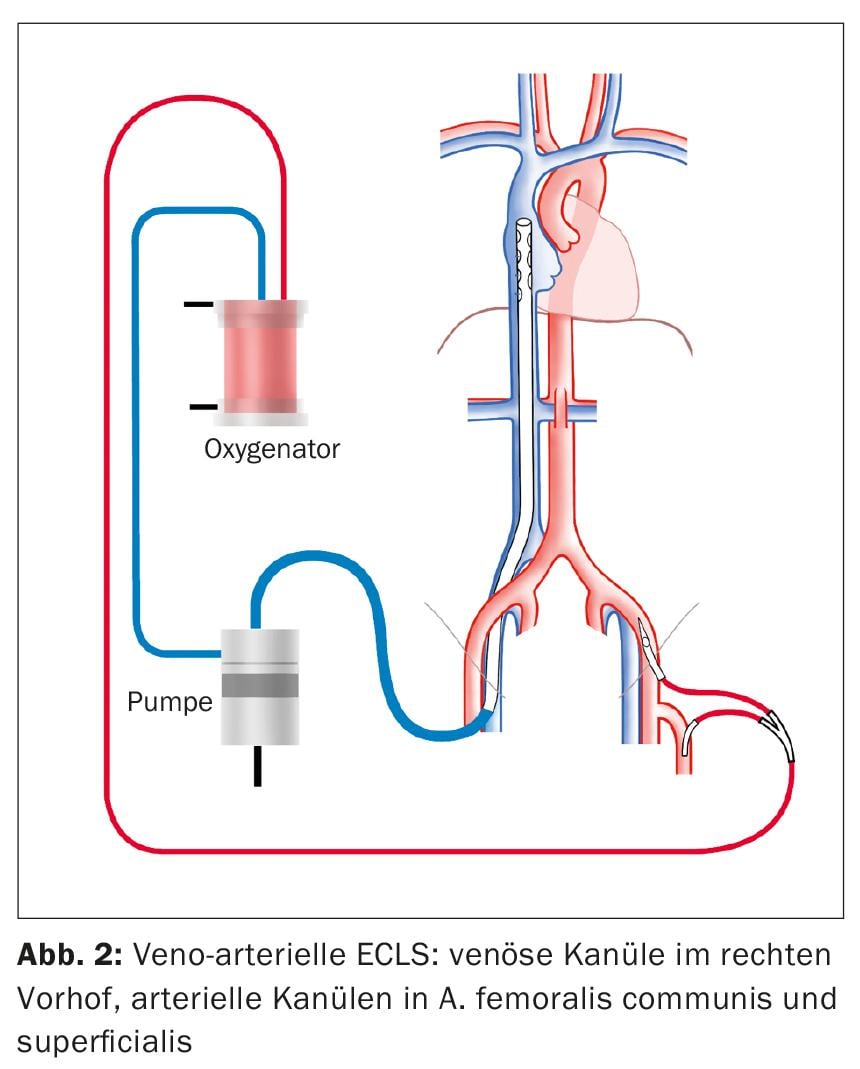
ECLS as a procedure for cardiogenic shock is implanted in a veno-arterial configuration (Fig. 2). Similar to vvECMO, a cannula is inserted into the right VFC for venous drainage. Also under echocardiographic control, the tip of the cannula is placed so that it lies 1-2 cm in the superior vena cava to achieve optimal drainage of the upper and lower half of the body. The standard access for arterial delivery is the common femoral artery (AFC). The cannula is inserted proximally into the external iliac artery. In addition, a perfusion leg is placed distally in the superficial femoral artery to prevent leg ischemia.
Primarily, all cannulas are implanted percutaneously. Prior to insertion of the AFC cannula, a percutaneous vascular occlusion system is placed to achieve occlusion of the puncture hole during subsequent percutaneous removal of the cannula. If percutaneous puncture of the AFC is unsuccessful, the cannula must be implanted openly by surgery. This procedure may be required for implantation under resuscitation conditions. No percutaneous vascular closure system is required for removal of venous cannulae from the VFC and VJI. A suture can be used to close the puncture hole.
Treatment strategies
vaECLS: Implantation of vaECLS often occurs in an emergency situation, which does not allow time to evaluate the treatment strategy after vaECLS. The patient’s comorbidities and psychosocial environment are usually unknown. However, this is a prerequisite for determining the treatment approach following vaECLS. Therefore, the vaECLS is usually implanted as a “bridge-to-decision” to allow time to determine the patient’s situation. In addition to clarification of concomitant diseases and the patient’s living situation, assessment of the neurological situation is required, especially if the vaECLS was implanted under resuscitation conditions. For this purpose, in addition to cranial CT and EEG, a wake-up test must be performed on ECMO to assess the patient clinically-neurologically. After completion of the examinations, four options for further therapy are available (Fig. 3):
- Weaning the patient off vaECLS if cardiac function recovers.
- If cardiac function does not recover: Switch the patient from vaECLS to a cardiac assist device as a “bridge-to-transplant” if the patient is a candidate for heart transplant, or as “destination therapy” if the patient is not a candidate for heart transplant.
- Heart transplantation if switching to a cardiac assist device does not seem reasonable, there are no contraindications to heart transplantation, and a suitable donor heart is available within a maximum of two to three weeks. For this, the patient is urgently listed on the waiting list. If a suitable donor heart is not available within this period, the patient will be implanted with a ventricular assist device, otherwise the complication rate at vaECLS will increase.
- Termination of vaECLS with consecutive death of the patient if the patient’s cardiac function does not recover and contraindications do not allow implantation of a cardiac assist device or heart transplantation.
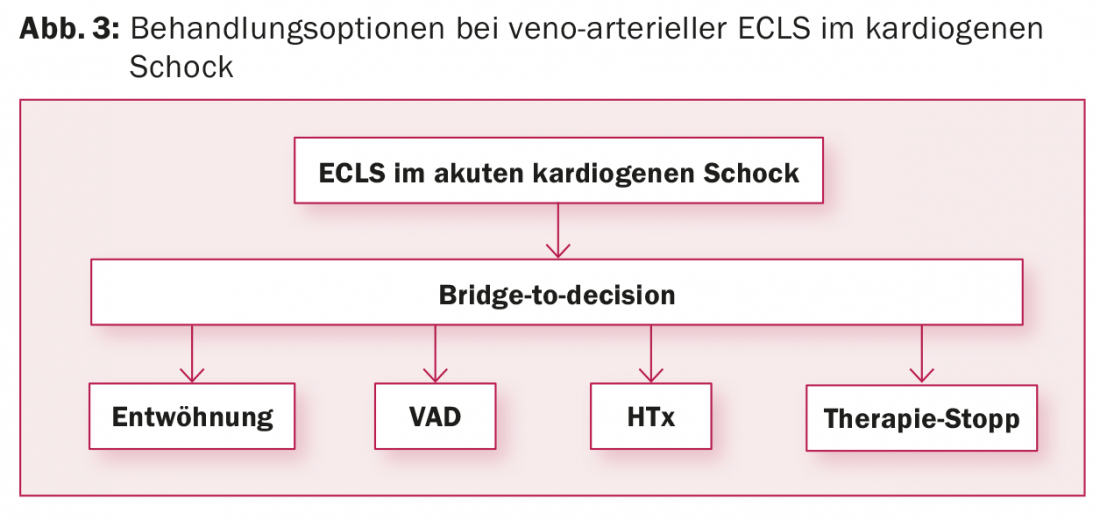
According to the current 2016 European Society of Cardiology guidelines for acute and chronic heart failure [3], implantation of a vaECLS in refractory cardiogenic shock can be considered taking into account patient age, comorbidities, and neurological function (recommendation IIb, level of evidence C).
vvECMO: Implantation of vvECMO is usually urgent and rarely emergency. There are three options as treatment options after vvECMO:
- Weaning from vvECMO in the event of pulmonary function recovery.
- Lung transplantation if lung function does not recover and there are no contraindications.
- Termination of vvECMO followed by patient death if lung function does not recover and the patient is not a candidate for lung transplantation.
Mortality and morbidity
vaECLS: The survival rate of patients who received vaECLS in cardiogenic shock to hospital discharge is approximately 40%. This is according to an analysis of 3846 patients from the Extracorporeal Life Support Organization (ELSO) International Registry from 2003 to 2013 [4]. Based on their results, the authors developed a risk score, the so-called “SAVE score”, which allows the estimation of the survival rate of patients at vaECLS depending on the risk factors present. A meta-analysis of 1199 patients from 22 observational studies from 2000 to 2014 confirms the ELSO registry analysis with a survival rate of vaECLS patients of 40% to hospital discharge [5]. The survival rate initially appears low at about 40%. However, 0% would have survived without vaECLS. The low survival rate despite restoration of a sufficient circulation by vaECLS is due, at least in part, to the complex processes involved in cardiogenic shock, with development of a systemic inflammatory response and multiorgand syndrome. Establishment of sufficient organ perfusion by vaECLS alone is obviously not always sufficient to break these processes.
Long-term survival rates of patients after implantation of a vaECLS are sparse in the literature. In the meta-analysis mentioned above, 3-year survival was reported to be 42.7% [5]. This is approximately the survival rate to hospital discharge. This means that mortality is only low once patients have been discharged from the hospital.
Complications on ECMO usually have an unfavorable impact on patient survival. More than half of all patients develop one or more complications [5]. Vascular complications may occur in 10-20% of patients [5,6]. Depending on the extent of vascular obstruction by the cannula in the AFC, leg ischemia may occur. To prevent this, a distal perfusion cannula is nowadays inserted into the AFS at the time of vaECLS implantation as standard. Bleeding complications occur in 26-41% of patients [5,6]. They are favored by the disseminated intravascular coagulopathy often present in cardiogenic shock, insufficient production of coagulation factors in hepatic insufficiency, thrombocytopenia, and blood thinning required at vaECLS.
Neurologic complications include ischemic or hemorrhagic stroke in 6-8% of patients [5,6]. They result from insufficient cerebral blood flow in cardiogenic shock or during resuscitation before vaECLS implantation. The complex coagulopathy resulting from cardiogenic shock, which is not always detectable by laboratory chemistry, may favor the development of these complications at the vaECLS. Infectious complications are common (25-49%) and include local infections at cannulation sites (17%) and systemic infections including pneumonia and sepsis. Sterility standards, which cannot always be maintained during emergency vaECLS implantation, and the compromised immune system in cardiogenic shock favor the development of infections [5–7]. Acute renal failure results from reduced perfusion in cardiogenic shock and occurs in 47-55% of patients [5,6]. Renal replacement is required in 40-46% of patients [6,7]. Risk scores have also been developed for vvECMO therapy to estimate the probability of survival, such as the PRESERVE or PRESET score [9,10].
vvECMO: More than 60% of patients receiving vvECMO for refractory respiratory failure survive to hospital discharge, according to a recently published meta-analysis of 1042 patients [8]. Patient age and insufficient cannula size with consequent insufficient oxygenation potential are risk factors.
Complications occur in 40.2%. Bleeding is the most common at 29.3%. Intracerebral hemorrhage occurs in 5.4% of cases. Bleeding at venous cannulation sites does not play a significant role at 10%, in contrast to the higher risk of bleeding at arterial cannulations in vaECLS. Local infections at the cannulation sites form in just under 10% of all patients. But only 7% of complications lead to death.
Transport of ECMO patients
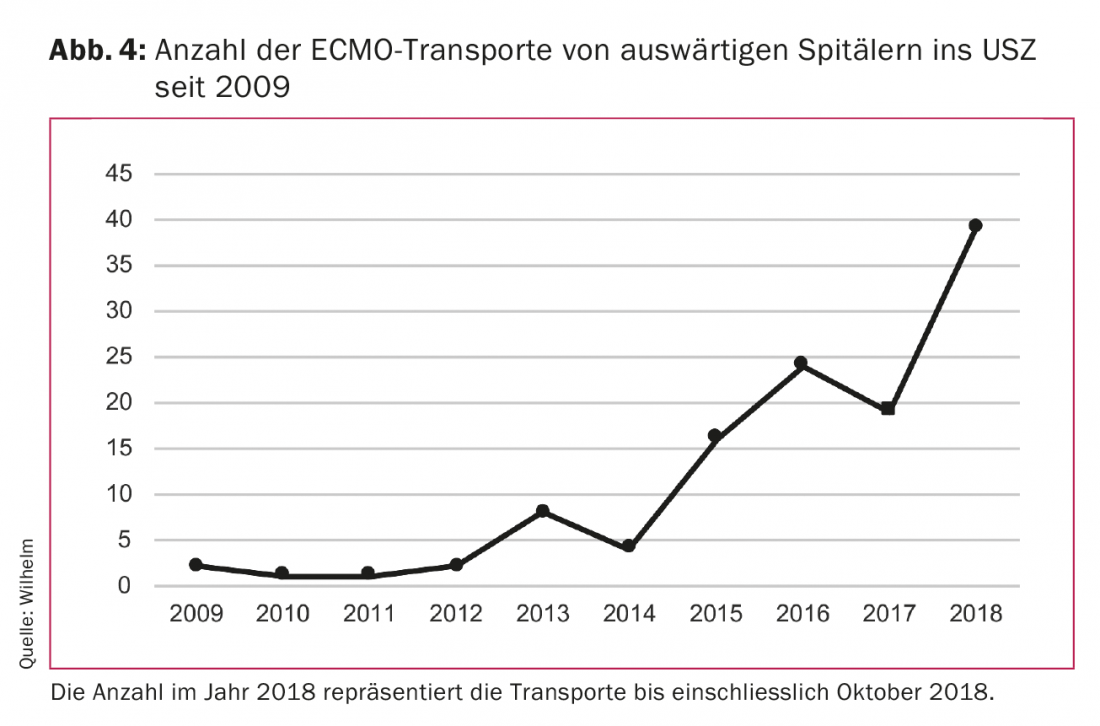
Technical advancements in centrifugal pumps and oxygenators have resulted in a significant reduction in the size of the systems. As a result, they can now easily be transported in an ambulance or helicopter. Thus, it has become possible to transport patients from hospital to hospital at the vaECLS or vvECMO. This has given rise to a new treatment concept. Patients in out-of-town hospitals with acute respiratory failure or in cardiogenic shock who are not transportable due to their critical condition can be cared for on-site with the vaECLS or vvECMO and subsequently transported on this system to the center hospital for further treatment. Our team from the Clinic for Cardiovascular Surgery at the University Hospital Zurich (USZ) was able to perform such transports successfully, safely and without technical problems ground-based or by helicopter. The need for this type of treatment has increased significantly in recent years (Fig. 4). The team, consisting of a cardiac surgeon and a perfusionist, is brought with the equipment to the out-of-town hospital, where they implant the vaECLS or vvECMO. The patient is then transported to the USZ on the system. Our results show that the survival rate of such patients is absolutely comparable to that of patients receiving vaECLS or vvECMO at our center hospital.
The challenge remains
The use of vaECLS in cardiogenic shock and vvECMO in refractory respiratory failure enables the therapy of critically ill patients who would have died before this treatment was available. However, this therapy is not yet as successful as we would like. This is related to the complexity of this patient population. In view of the human, material and financial effort involved, the use of vaECLS or vvECMO must be critically examined in each individual case from an ethical and socioeconomic point of view. The use of scores can be an aid to risk assessment here. Randomized-controlled trials, which can provide guidance in other areas of medicine, are not yet available as guides in this field and may never be available.
Take-Home Messages
- Veno-venous ECMO is used in refractory respiratory failure.
- Veno-arterial ECLS is implanted in acute cardiogenic shock.
- Veno-venous ECMO and veno-arterial ECLS serve as “bridge-to-decision.”
- Survival to hospital discharge is 40% for vaECLS and 60% for vvECMO.
- Patients can be safely transported from the out-of-town hospital to the center hospital at the vaECLS or at the vvECMO.
Literature:
- Peek GJ, et al: Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009; 374(9698): 1351-1363.
- Thiele H, et al: Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013; 382(9905): 1638-1645.
- Ponikowski P, et al: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27): 2129-2200.
- Schmidt M, et al: Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE) score. Eur Heart J 2015; 36(33): 2246-2256.
- Xie A, et al: Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest: A meta-analysis. J Cardiothorac Vasc Anesth 2015; 29(3): 637-645.
- Cheng R, et al: Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014; 97(2): 610-616.
- Bermudez CA, et al: Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg 2011; 92(6): 2125-2131.
- Vaquer S, et al: Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care 2017; 7(1): 51.
- Schmidt M, et al: The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013; 39(10): 1704-1713.
- Hilder M, et al: Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Crit Care 2017; 21: 301.
CARDIOVASC 2018; 17(6): 4-7