There are new therapeutic approaches in multiple myeloma. For example, the efficacy and safety of CAR-T cells are being studied. In particular, they could be applicable in the refractory and relapsed situation in the future.
Lenalidomide is increasingly used for induction therapy in patients with multiple myeloma. However, due to lenalidomide resistance in multiple myeloma, which frequently occurs in the further course of treatment, the search for effective follow-up therapies is medically necessary.
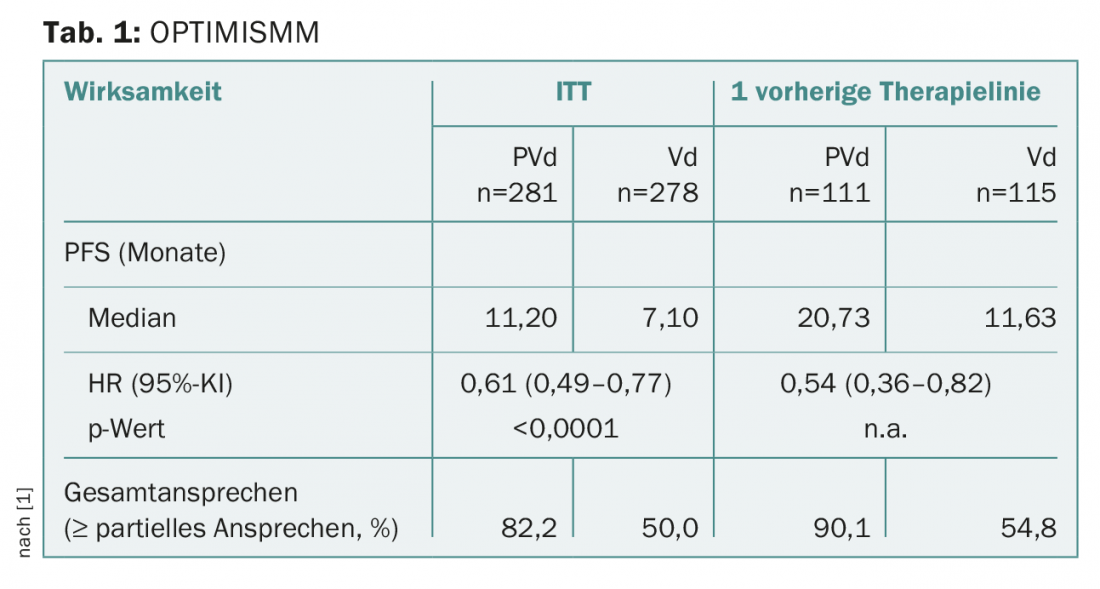
OPTIMISMM: Proven to be more than just optimism
Nowadays, pomalidomide is usually used in the treatment of relapsed/refractory multiple myeloma, and was even often still shown to be effective against lenalidomide-resistant cells [1]. The phase III study presented called OPTIMISMM in lenalidomide-pretreated patients with relapsed/refractory multiple myeloma (of whom about 70% were previously refractory to lenalidomide) revealed, among other things, a significant prolongation of progression-free survival: In the intention-to-treat population, but also after only one prior treatment (including lenalidomide), both PFS and overall response rate (ORR) were better for pomalidomide, bortezomib, and low-dose dexamethasone compared to bortezomib and dexamethasone alone (Table 1) [1].
Sustained treatment response thanks to immunotherapy?
In addition, at this year’s EHA congress, another novel treatment approach for multiple myeloma was discussed very lively, immuno-oncology using CAR-T cells. Especially in very heavily pretreated, refractory MM patients, immunotherapy could represent a treatment option in the future.
As a therapeutic target, the B-cell maturation antigen (BCMA for short) currently appears to be very promising. BCMA is predominantly expressed on the surface of plasma cells and is found in very few other tissues of the human body. This makes it a suitable tumor marker and potential target for immunotherapy [2].
In an initial phase I multicenter trial [2], hematologists are using bb2121, second-generation chimeric antigen receptor (CAR) T cells that can recognize BCMA with pinpoint accuracy. The dose-escalation phase of the study has already provided promising results regarding efficacy and safety of bb2121 in patients with relapsed/refractory multiple myeloma.
Just over half of the patients presented participated in the escalation phase. Previously, they had to have undergone at least three therapies (including a proteasome inhibitor and immunomodulator) or be dually refractory to therapy. On their plasma cells, these patients carried BCMA in ≥50% of cases [2]. The second part of the ongoing study, the so-called dose-expansion phase, called for daratumumab pre-therapy for the remaining patients presented. In addition, the disease had to be refractory to the last therapy. In contrast, proven BCMA expression was no longer required.
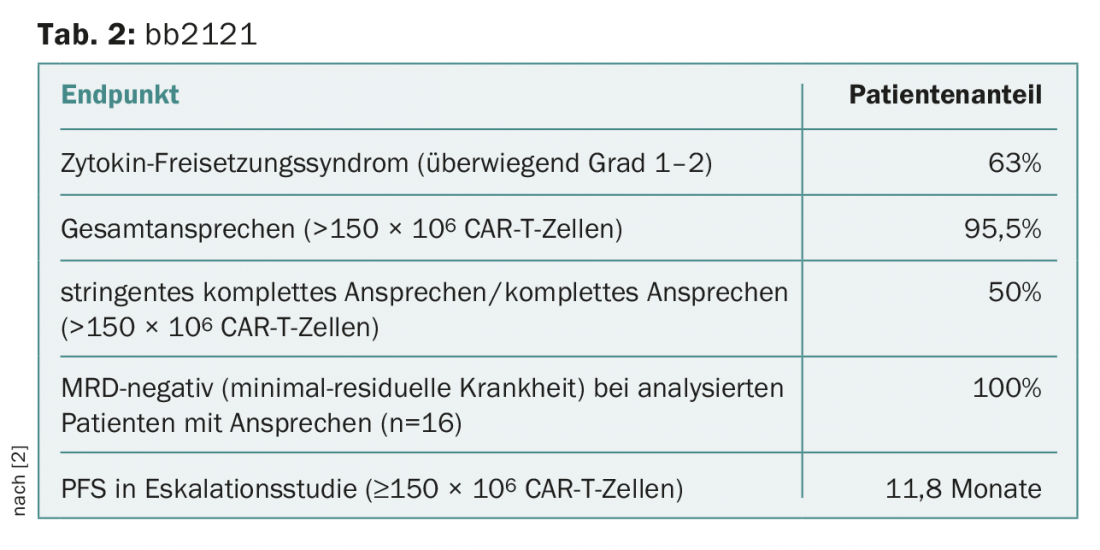
As a result, bb2121 showed great promise at doses of ≥150 × 106 CAR-T cells (Table 2) [2]:
- There was a sustained response to therapy, and neurotoxicity and cytokine release syndrome (CRS) were controllable as mainly grade 1-2.
- Overall, CAR T-cell therapy against BCMA with bb2121 certainly appears to represent a new treatment option for patients with relapsed/refractory multiple myeloma.
Hot Topic: Immunotherapy
Multiple myeloma is genetically very heterogeneous and therefore a complex disease. Today, next generation sequencing (NGS) already goes well beyond the diagnostic power of conventional cytogenetics and has helped, among other things, to decipher important signaling pathways and mutational processes that can influence the prognosis of a patient with multiple myeloma. At the same time, advances within genetic diagnostics are the starting point for new therapeutic procedures such as immunotherapy, which of course includes the CAR-T cell procedure or the anti-CD38 antibody Daratumumab.
The anti-CD38 monoclonal antibody daratumumab is already an important factor in the fight against multiple myeloma. The mechanism of action is as simple as it is effective: The anti-CD38 antibody induces cell death of the myeloma cells on the one hand by direct immune effects and on the other hand by the elimination of immunosuppressive cells. Studies further showed that combinations of monoclonal antibodies such as daratumumab or elotuzumab and immunomodulatory drugs or proteasome inhibitors can increase their therapeutic efficacy but without leading to more toxic effects.
In the future, the range of immunotherapies for multiple myeloma is expected to increase even further, experts said at the EHA congress. For example, conjugates consisting of an antibody and a drug or the use of checkpoint inhibitors for the treatment of multiple myeloma are discussed [3].
Hematologists and oncologists should keep this in mind
In recent years, there have been some remarkable developments in the field of therapeutic options for multiple myeloma. Nevertheless, it should not be forgotten that MM is still a non-curable tumor disease today. Especially in relapsed/refractory multiple myeloma, the choice of therapeutic options is considered limited. Immunotherapy may offer new opportunities in this regard in the future, however, many approaches to the treatment of MM, including CAR-T cells, are currently still in the development phase and are thus unfortunately not yet available for everyday practice.
The approval of various new combinations of drugs (e.g., carfilzomib-lenalidomide-dexamethasone, daratumumab-lenalidomide-dexamethasone or elotuzumab-lenalidomide-dexamethasone, and bortezomib-dexamethasone-daratumumab) has indeed improved therapy for patients with relapsed/refractory multiple myeloma. However, this also complicated the therapeutic decision-making process on the part of physicians and patients. In any case, therapy must be optimized for an individual patient, especially in the case of recurrence [3].
Source: EHA Congress, June 14-17, 2018, Stockholm.
Literature:
- Richardson P, et al: OPTIMISMM: phase 3 trial of pomalidomide, bortezomib, and low-dose dexamethasone vs bortezomib and low-dose dexamethasone in lenalidomide-exposed patients with relapsed/refractory multiple myeloma. EHA 2018; Abstract S847.
- Raje N, et al: bb2121 anti-bcma car t cell therapy in patients with relapsed/refractory multiple myeloma: updated results from a multicenter phase I study. EHA 2018; Abstract S138.
- Van de Donk N: Immunotherapy in myeloma: why, when and how? Topic: 3ec Plasma Cell Myeloma (Multiple Myeloma). EHA 2018; 219162.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(4): 39-40.