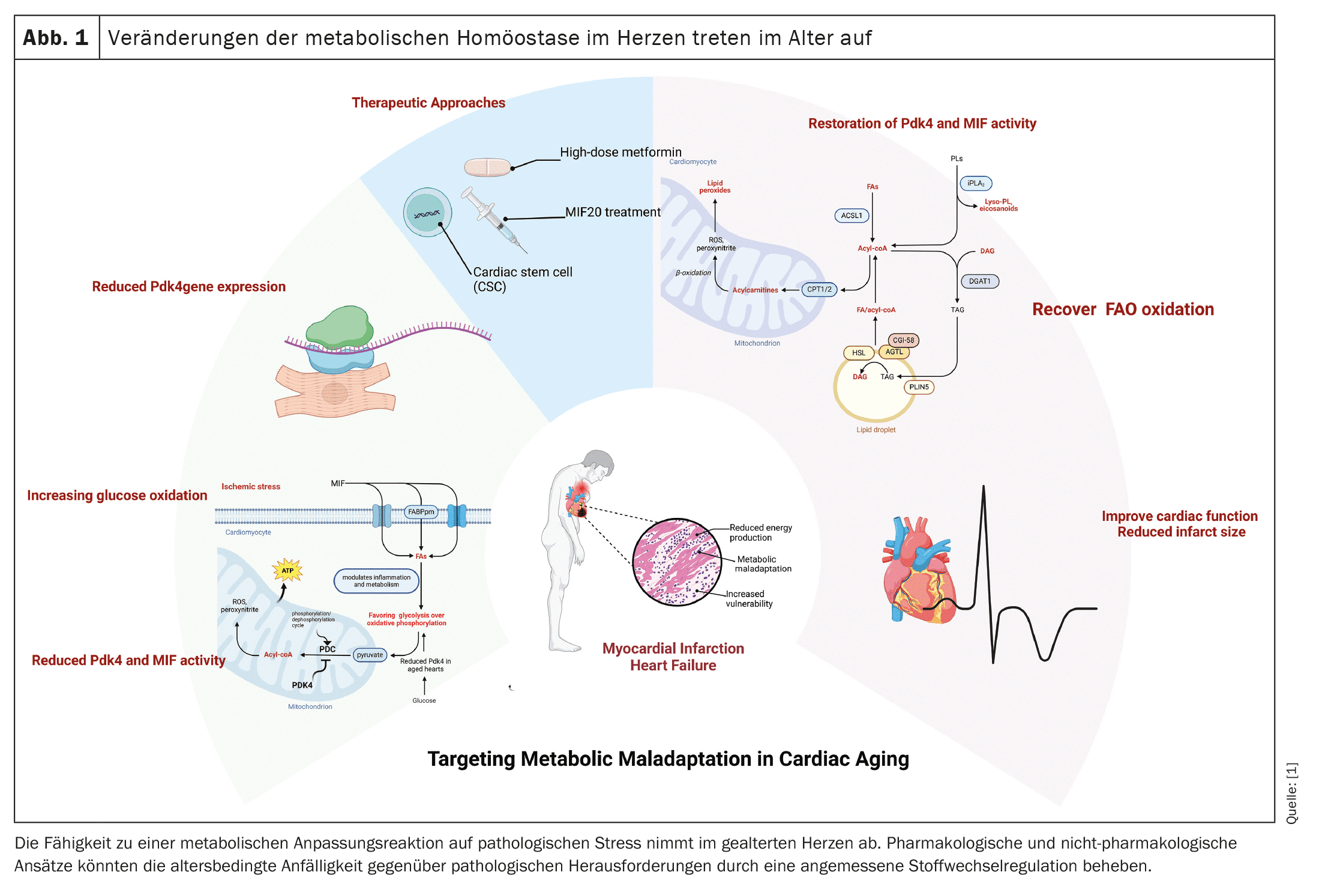
Cardiovascular disease, particularly coronary artery disease (CAD) and heart failure (HF), is common in the ageing population and is one of the leading causes of death worldwide. With age, the heart’s ability to recover from the stresses of myocardial infarction (MI) decreases, leading to significant changes in energy metabolism and ultimately contributing to heart failure. Understanding the mechanisms underlying these processes is crucial to developing new targeted therapies.
Metabolic changes after a myocardial infarction
(red) After myocardial infarction, the aged heart undergoes significant metabolic changes that impair its ability to contract and provide adequate cardiac output. During the anaerobic conditions of myocardial infarction, the heart switches from fatty acid oxidation (FAO) to less efficient glucose oxidation. Our studies in mouse models have shown that the left ventricular tissue of older animals after myocardial infarction continues to rely on glucose oxidation, which is regulated by pyruvate dehydrogenase kinase 4 (Pdk4). This mismatch in left ventricular cardiomyocytes after myocardial infarction signals a reduced ability of the aged heart to restore cardiac output, ultimately leading to heart failure.
Pdk4 plays a key role in cellular energy metabolism by regulating the pyruvate dehydrogenase complex (PDC), which converts pyruvate to acetyl-CoA, linking glycolysis to the tricarboxylic acid cycle (TCA) and oxidative phosphorylation. In an aged heart, decreased expression of Pdk4 leads to a shift from fatty acid oxidation to chronic glucose oxidation, which is less efficient in ATP production. This metabolic switch is particularly detrimental after myocardial infarction, as the heart’s energy needs are not met, further impairing cardiac function and contributing to heart failure.
The role of macrophage migration inhibitory factor (MIF)
In addition to Pdk4, macrophage migration inhibitory factor (MIF), which modulates inflammation and metabolism, is also decreased in the aged heart and attenuates the adaptive response to ischemic stress. Up-regulation of Pdk4 has been shown in studies to enhance fatty acid oxidation and improve cardiac performance in aged myocardium, making Pdk4 a potential therapeutic target in post-MI cardiac dysfunction. One promising therapy is the use of MIF agonists such as MIF20, which restore MIF signaling after ischemia, minimize infarction, and revitalize cardiomyocyte metabolism by modulating Pdk4. MIF20 may help correct the maladaptive response of Pdk4, allowing aged cardiomyocytes to return to fatty acid oxidation and improve cardiac performance.
Infarct size and its significance for the cardiometabolic state
In addition to the metabolic conversion following a myocardial infarction, the size of the infarct is an important factor in the recovery of cardiac function. Studies have shown that there is a strong correlation between impaired cardiomyocyte function and infarct size. For example, administration of a high dose of metformin during the reperfusion phase has been shown to reduce infarct size, improve contractility and positively influence cardiomyocyte metabolism in both mice and humans. Non-invasive treatment approaches such as cardiac stem cell therapy (CSC) have also shown promising results in reducing infarct size and improving cardiac performance.
Advances in heart repair: from protein therapies to CRISPR and exosomes
The development of innovative therapeutic approaches for the treatment of post-MI heart failure is progressing steadily. In addition to protein therapies such as MIF20, new approaches such as CRISPR gene editing to correct heart failure-causing genes and exosome therapies to promote tissue repair are also being actively researched. In addition, clinical trials on stem cell therapy are currently being conducted with the aim of regenerating damaged heart tissue.
Limiting the consequences of infarction is critical to improving health outcomes in patients with CAD. Left ventricular systolic dysfunction (LVSD) and heart failure are common consequences of myocardial infarction. While invasive treatments such as coronary bypass surgery can improve left ventricular dysfunction, right ventricular function often deteriorates further, contributing to worsening heart failure. Therefore, non-invasive approaches that both reduce infarct size and improve cardiac metabolism are of particular importance.
Future prospects and clinical relevance
Given the increasing incidence of CAD worldwide, preventing progression to heart failure remains critical. Understanding the metabolic maladaptations following myocardial infarction and developing new therapies to restore cardiac energy regulation could significantly improve patients’ quality of life. In this context, protein therapies and pharmacological interventions that optimize the energy metabolism of the heart are promising approaches.
Overall, the detailed mechanistic insights into the role of Pdk4 and MIF in cardiac metabolism in the elderly offer promising therapeutic approaches to improve cardiac function in elderly patients after myocardial infarction. Future studies should focus on further refining these therapies and evaluating their efficacy in clinical applications.
Source:
- Fatmi MK, Rouhi N, Lozonschi L, Li J: Cardiac metabolism in the elderly: effects and consequences. Aging (Albany NY). 2024 Aug 19; 16(16): 11773-11775. doi: 10.18632/aging.206071. Epub 2024 Aug 19. PMID: 39167437; PMCID: PMC11386932.
CARDIOVASC 2024; 23(3): 38-39