Two antifibrotic drugs are currently available for the drug therapy of idiopathic pulmonary fibrosis (IPF): Pirfenidone and the tyrosine kinase inhibitor nintedanib. Therapy intolerances are rare, but do occur. However, the option of switching between the two agents in such cases has been little explored.
Both pirfenidone and nintedanib are generally well tolerated by patients, but side effects can lead to dose reduction, therapy interruption, and even therapy discontinuation or change. Lena Piotraschke of the Department of Internal Medicine and Pneumology at Fachkrankenhaus Coswig (D) and her colleagues retrospectively analyzed data from 284 patients treated for diagnosed IPF at Fachkrankenhaus Coswig between 2013 and 2019. 83% of them were male, and the average age was 71.6 years with an age range of 44 to 89 years.
Antifibrotic therapy with pirfenidone or nintedanib was given to 234 (82.4%) patients (5.6% received other therapy, 12% no therapy at all), of whom 209 (73.6%) presented at least at one follow-up visit. From this collective, 124 patients (59.3%) initially started pirfenidone therapy, of whom 30 (24.1%) later switched to nintedanib. Eighty-five patients (40.6%) started nintedanib in reverse, of whom 5 (5.8%) switched to pirfenidone during the observation period. Overall, this meant that 12.3% of patients changed their antifibrotic therapy. Transient dose reduction occurred in 17% of patients treated exclusively with pirfenidone and in 33.8% of patients treated with nintedanib.
Side effects are the order of the day
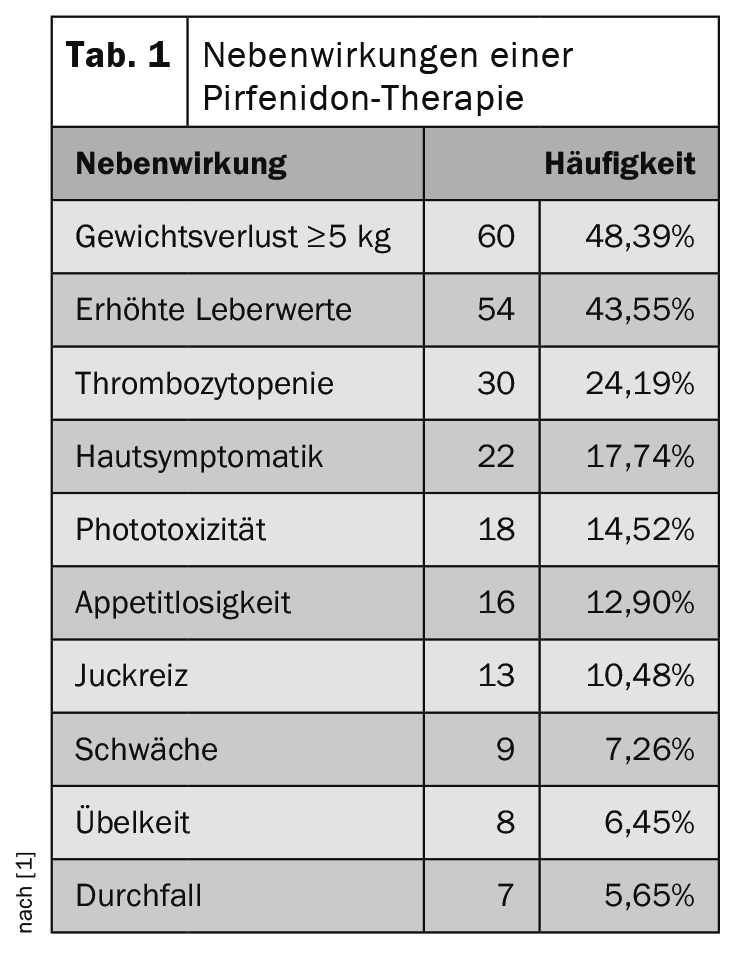
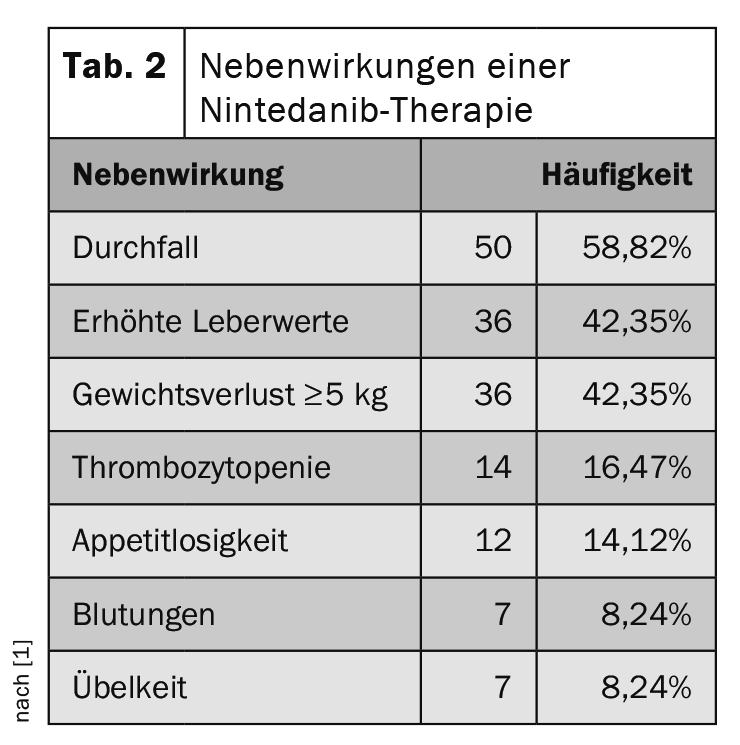
Almost 90% of patients reported side effects during the observation period. In addition to weight loss and elevated liver enzymes, the main side effects of pirfenidone are an increased frequency of skin symptoms and phototoxicity (table 1). In comparison, nintedanib often has a gastrointestinal side effect profile (Tab. 2) .
Causes for modification of therapy, i.e., dose reduction, short-term interruption of therapy, or change of therapy, were primarily side effects (88.3% for pirfenidone, 95.8% for nintedanib). The speaker explained that other reasons for a switch could be identified as well as a lack of success in therapy, but also other, e.g. personal reasons. Complete treatment discontinuations were also primarily due to adverse events (79.1% for pirfenidone, 92.3% for nintedanib).
During the course of treatment, a total of 76.7% of patients who switched antifibrotic medication from pirfenidone to nintedanib reported a subjective improvement in general well-being. Likewise, such improvement was documented in 60% of patients who switched their therapy from nintedanib to pirfenidone. Only 10% of patients discontinued therapy altogether after switching to nintedanib due to intolerance.
Antifibrotic therapy with pirfenidone and nintedanib is recommended and established according to evidence-based criteria. Since side effects are the most common cause for a necessary therapy modification, they should be well observed and treated. It is also shown that a change of therapy after previous problems is a good option and should be clinically recommended and established in any case to improve the patients’ quality of life and to achieve a therapeutic success, the speaker concluded.
Congress: DGP 2021 digital
Source:
- E-Poster “Clinical experience with antifibrotic therapy in patients with idiopathic pulmonary fibrosis (IPF) with special reference to change of therapy at a tertiary pulmonary center”. 61st Congress of the German Society for Pneumology and Respiratory Medicine e.V., 2.6.2021.
InFo PNEUMOLOGY & ALLERGOLOGY 2021; 3(3): 2 (published 9/16-21, ahead of print).