Due to its special protective function for the body, the skin is an exception in the aging process: As a mechanical and biological boundary between internal organs and the environment, the skin is additionally exposed on a daily basis to a multitude of exogenous influences that act not only once and directly (injury), but also repetitively and with great latency. For this reason, a distinction is made between intrinsic and extrinsic aging in the skin.
Intrinsic aging is often also referred to as chronological or genetic aging, extrinsic aging as exogenous or premature aging, and, if ultraviolet light is the exogenous noxious agent, also as photoaging. Morphologically, intrinsic skin aging cannot always be sharply separated from extrinsic aging. The two processes run in parallel, and extrinsic aging of the skin can override intrinsic aging to varying degrees in different parts of the body.
Since the mechanisms underlying intrinsic and extrinsic aging are similar and the biochemical, morphological, and clinical characteristics of skin aging can be directly derived from the pathomechanisms, some specific aspects will be presented first. Some extrinsic factors are highlighted separately.
The importance of reactive oxygen species [1, 2].
Reactive oxygen species (ROS) play a central role in skin aging. According to the free radical theory of aging formulated by Harman [1], the formation of such molecules occurs continuously throughout life. This occurs particularly in tissues with high oxygen turnover, such as brain, muscle and liver.
At the cellular level, mitochondria are the site with the highest turnover of ROS. In the course of energy production by the respiratory chain, H2O2 is formed at the inner mitochondrial membrane. This is also produced during light aging, where it is generated from superoxide anions by superoxide dismutases. A large number of studies have shown that in the context of extrinsic aging, H2O2 and singlet oxygen are the main ROS induced by UVA light. Hydroxyl radicals, on the other hand, are formed together with H2O2 as part of the physiological Fenton reaction and are the main oxygen radicals in extrinsic skin aging in the context of UVB exposure.
ROS are thus central to both intrinsic and extrinsic aging of the skin.
In the course of evolution, the organism has developed several defense mechanisms against ROS. These include catalase, cytochrome p450, glutathione, etc. When the capacity of the body’s own systems to neutralize ROS is exceeded, the highly reactive molecules can react with various components of the cell and damage them. For example, cellular macromolecules such as DNA, proteins and lipoproteins can be directly damaged. Overloading of endogenous defense mechanisms by continuous ROS formation leads to gene mutations and protracts skin aging.
Based on the free radical theory of aging, attempts are now being made among large – especially Caucasian – populations to limit or even prevent ROS formation systemically through dietary supplements and topically through the application of products containing natural and synthetic antioxidants. Unfortunately, it has been shown that the Harman experiments cannot be fully replicated and that, depending on the experimental setup in worms and mice, an increased occurrence of ROS may even lead to a prolongation of lifespan [3]. It is postulated that ROS initiate a cellular repair network. In fact, it is now believed that the intake of high doses of antioxidants in the form of vitamins and other supplements may not do anything or may even do harm.
Mitochondrial DNA mutation [4, 5].
The main task of mitochondria is to provide energy in the form of ATP. This occurs through the respiratory chain, which takes place at the inner mitochondrial membrane. This process is not always error-free. Errors in the respiratory chain can lead to the generation of ROS, which is why the mitochondrion is the site with the highest turnover of ROS in the cell.
Mitochondria contain their own genetic material (mtDNA). The mtDNA is located in close proximity to the respiratory chain and thus in close proximity to the damaging effects of the nascent ROS. It has been shown that mutations of mtDNA accumulate in the normal aging process and that respiratory chain function decreases in inverse proportion, leading to a decrease in energy provision (mitochondrial theory of aging). Furthermore, it has been shown that beyond the intrinsic aging mentioned above, mutations of mtDNA also play a role in extrinsic aging of the skin (mitochondrial theory of photoaging). Skin repetitively exposed to UV light showed induction of mutations of mtDNA leading to functional disorders of mitochondria (oxygen consumption, mt membrane potential, energy metabolism).
Matrix metalloproteinases [6, 7].
Marixmetalloproteinases (MMPs) represent a family of enzymes whose function is to proteolytically degrade dermal matrix proteins. The main substrate of MMPs are structural proteins of the dermis, such as collagens and gelatin. The function of MMPs is important in the context of tissue remodeling that occurs in various biological processes such as morphogenesis, wound healing, angiogenesis, or tumor growth. In addition, MMPs have other functions in processing signaling molecules that control cell behavior. Physiologically, tissue-specific inhibitors of matrix metalloproteinases (TIMP) exist for MMPs. They reduce the activity of MMPs and thus curb, for example, excessive protein degradation. The interaction of the MMP and the TIMP is not yet fully understood.
A large body of work suggests that MMPs play an important role in extrinsic skin aging. MMPs are induced directly and indirectly by ultraviolet light. The degradation of dermal matrix proteins induced in this way leads to a change in the extracellular matrix that contributes to the clinically and histologically visible changes characteristic of photoaged skin.
Studies indicate that retinoids have an inhibitory effect on the expression of MMPs. Accumulation of new, unfractionated collagen can subsequently lead to a significant improvement in the appearance of the skin.
Activation of transcription factors [8, 9].
Activation of transcription factors by UV light leads to the induction of MMP. UV exposure of human skin leads not only directly to the induction of MMP, but also to the induction of the transcription factors AP-1 and NF-κB. These factors are activators of MMP genes. This pathway of indirect MMP activation in extrinsically aged skin exists in addition to direct MMP induction by ROS.
Results from laboratory and animal experiments indicate that the induction of transcription factors can be inhibited with plant extracts (magnolol).
“Advanced Glycation Endproducts” [10, 11].
“Advanced Glycation Endproducts” (AGEs) are formed as part of a Maillard reaction by glycation and oxidation of various structural proteins. In this process, proteins (free amino groups) or lipids react non-enzymatically with carbohydrates. AGEs are involved in various diseases of aging. Glycation is involved in both intrinsic and extrinsic (UV exposure) skin aging and alters growth and differentiation processes and enzyme activities (MMP) in the extracellular matrix of the skin. The increased content of AGEs in the skin alters the mechanical properties. They are characterized by reduced elasticity and increased stiffness.
Results from laboratory studies indicate that the formation of AGEs can be inhibited with plant extracts (red grapefruit).
Vitamin D [12–15]
Vitamin D is a fat-soluble vitamin that is primarily formed in the human skin under sunlight (especially UV-B). Among other things, it regulates calcium and phosphate absorption from the intestine and their incorporation into the bones. The age-related decrease in skin thickness results in significantly reduced vitamin D synthesis in the skin. In addition, in older, less mobile people, reduced sun exposure negatively affects vitamin D status.
The importance of vitamin D has received great attention in recent years and is currently the subject of lively debate. The systematic review by Philippe Autier [15], 6/12/13, clearly shows that the opinions frequently circulated about vitamin D should be differentiated. Many prospective studies have shown an association between low 25(OH)vitamin D concentrations and a number of acute and chronic diseases. However, a similar number of randomized trials have failed to provide evidence that increasing vitamin D concentrations prevents occurrence.
Immune senescence [16]
Even in apparently healthy people, the aging immune system undergoes profound and sometimes irreversible changes that lead to a progressive reduction in immune function – to immune senescence. This affects all organs and cells of the innate and acquired immune system, including hematopoietic stem cells (HSC), lymphoid progenitor cells (CLP) in bone marrow and thymus, mature T and B lymphocytes in secondary lymphoid organs, as well as phagocytes and antigen presenting cells (APC) of the innate immune system. At the level of the whole organism, these age-associated changes lead primarily to an increased tendency to infection and to a reduced response to vaccinations. Tumor and autoimmune diseases also occur more frequently. Numerous of these affect the dermatologic specialty such as epithelial carcinomas, malignant melanoma, cutaneous lymphomas, collagenoses, bullous dermatoses, herpes zoster, wound healing disorders, and others.
Aging of innate immunity: The cells of the innate immune system are in the forefront of defense against pathogens. These include in particular the phagocytic and/or antigen-presenting cells such as neutrophils, macrophages and dendritic cells (DC), but also those on the borderline of acquired immunity such as NK cells. Some of the age-related changes in the innate immune system are summarized in Table 1 .
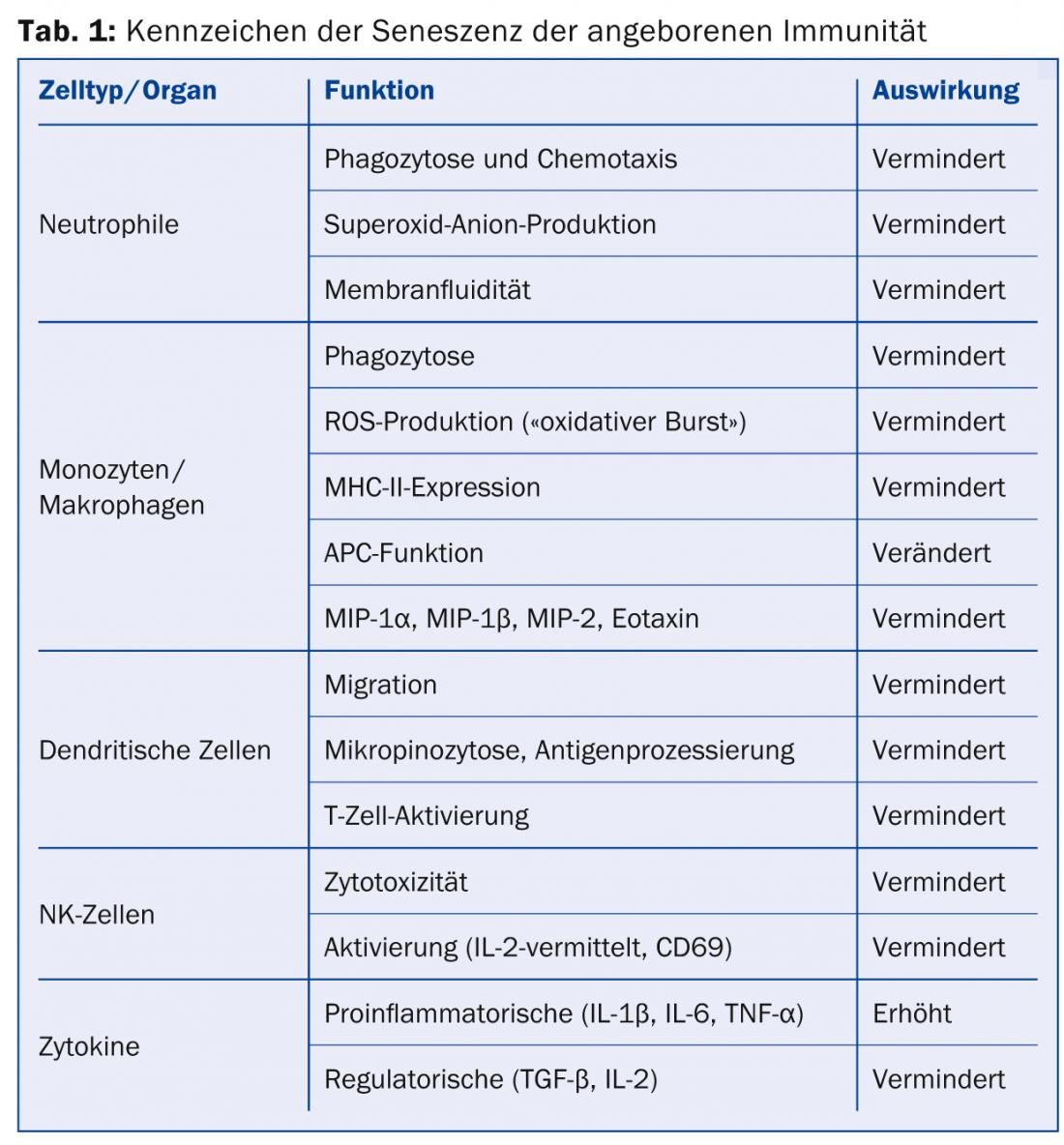
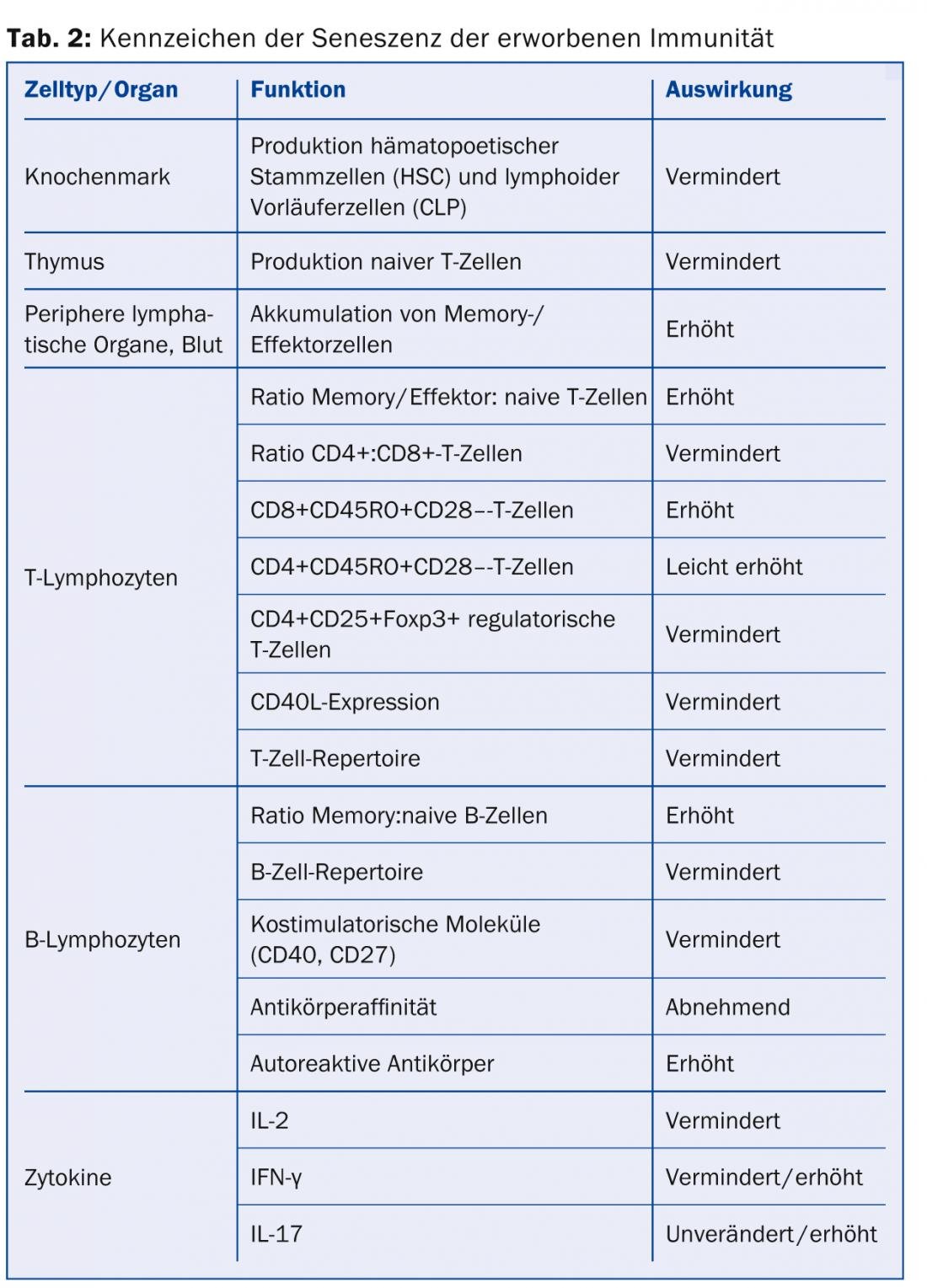
Aging of acquired immunity: In contrast to innate immunity, acquired immunity is capable of antigen-specific immune responses, mainly through T and B cells. These are also subject to age-related changes that, as with innate immunity, result in reduced (or misdirected) striking power against pathogens. In addition, however, acquired immunity also results in reduced variability and thus reduced adaptability to antigens (Table 2).
Environmental changes of the skin
Environmental changes of the skin in us today are primarily caused by smoking and ultraviolet radiation (UV-S).
Smoking [17, 18]: The impairment of skin physiological processes by smoking is well studied. Smoking generally impairs the blood circulation of tissues, including that of the skin. The skin of smokers is often sallow and grayish, it is also drier and flabbier and becomes wrinkled earlier and more severely. The phenomena have been impressively documented in some work in identical twins. These phenomena appear earlier and more clearly in women than in men. Several skin physiological processes, which are affected by the numerous free radicals in tobacco smoke, are responsible for the increased formation of wrinkles in smokers. Thus, tobacco smoke inhibits the formation of new collagen fibers in the dermis and at the same time promotes the breakdown of collagen and elastin fibers. The finely tuned system of building up and breaking down the fibers is thus thrown out of balance. UV exposure further impairs these processes. In addition, nicotine from tobacco smoke constricts blood vessels so that the skin also receives insufficient blood flow, and carbon monoxide reduces oxygen transport through erythrocytes.
UV exposure [19–22]: The impairment of skin physiological processes, especially by extensive UV exposure, is also well studied (Fig. 1).

This was illustrated particularly impressively last year in a paper by Jennifer Gordon in the New England Journal of Medicine [20]. The clinical presentation shows a 69-year-old truck driver whose left-sided facial skin thickened and wrinkled asymptomatically over a period of 25 years of work and showed typical signs of UV-A associated skin damage. To date, little is known about epidermal light aging, and the molecular processes that lead to pigmentary disorders – typical of light-damaged skin – are not yet well understood. Dermal light aging is far better studied. Due to increased matrix metalloproteinase, collagenase, 92-kd-gelatinase and stromelysin activity, collagen is increasingly degraded with age. Even small or intermittent amounts of UV light activate matrix metalloproteinase and lead to degradation of collagen and inhibition of collagen synthesis. This is also where treatment with retinoic acid comes in to stop UV-induced matrix metalloproteinase activation. Similar processes are also described for elastin. The interaction of these processes leads to the typical light-induced wrinkling.
Meanwhile, work is also available that powerfully describes the role of environmental pollution in skin aging. But smoking and UV-S are not the only environmental factors. Gravity, facial expressions and sleeping position are also extrinsic factors that make a not inconsiderable contribution to the visible changes in aging skin: Gravity tugs ceaselessly at our bodies and its signs become particularly apparent after the age of 50. The nose, ears, eyelids and lips are particularly affected. Nasolabial, marionette and neck wrinkles are categorized as gravitational wrinkles and occur due to sagging skin and subcutaneous tissue.
Facial expressions cause more and more wrinkles to form as we age. Mimic wrinkles can appear very early in the course of overactivity of the facial muscles. Roughly, they can be divided into crow’s feet, frown lines, forehead lines, as well as mouth, lip or smoker’s lines, as well as chin or nose lines.
Certain sleeping positions lead to the typical sleep wrinkles, which usually run vertically in the area of the cheek and temples.
Large ethnic differences can be observed in age-related wrinkling. In Japan and South Korea, for example, women remain virtually wrinkle-free until the age of 50, while in France, expression lines become visible from the age of 20 to 30. After the age of 50, however, the situation turns in favor of European women again.
Genetic engineering and skin care [23]
In recent years, great advances in genetic engineering and the development of “gene chips” have given us a deeper insight into the molecular events of skin aging. Gene expression analyses led to the identification of signaling pathways involved in the aging process, and this knowledge can undoubtedly contribute to the development of new strategies for prevention and therapy.
However, the often enthusiastic reports on the effect of new (sometimes old) ingredients on the aging process have been conducted in vitro, and translation to “clinical” everyday situations is still pending. These active ingredient candidates are nevertheless often offered in homeopathic dosages rich in words and colors in so-called highly effective and innovative cosmetics. The hype surrounding stem cells has been particularly impressive since Michelle Obama started treating her face with a serum containing stem cells from the long-lasting Swiss apple variety “Uttwiler Spätlauber”. Thus, the consumer – supported by a lot of celebrities – is suggested that with these products the stem cells of the skin are reminded again of their task to make themselves useful and to rejuvenate the skin.
Conclusion for practice
- Intrinsic aging is often referred to as chronological or genetic aging, while extrinsic aging is often referred to as exogenous or premature aging.
- Reactive oxygen species (ROS) are central to both intrinsic and extrinsic aging of the skin.
- Mutations of mitochondrial DNA accumulate in the normal aging process. The function of the respiratory chain decreases in inverse proportion to this, leading to a decrease in energy provision (mitochondrial theory of aging).
- Smoking, UV-S, gravity, facial expression and sleeping position are considered environmental factors for skin aging.
Prof. Dr. phil. nat. Christian Surber
Literature:
- Harman D: J Gerontol 1956; 11: 298-30000. PMID 13332224.
- Fortmann SP, et al: Ann Intern Med 2013; 159(12): 824-834. doi:10.7326/0003-4819-159-12-201312170-00729.
- Moyer MW: Sci Am 2013 Feb; 308(2): 62-67. PMID: 23367786.
- Berneburg M, et al: Photochem Photobiol Sci 2006; 5: 190-198. PMID: 16465305.
- Kazachkova N, et al: Aging Dis 2013; 4(6): 337-350. PMID: 24307967.
- Griffiths CE, et al: N Engl J Med 1993; 329(8): 530-535. PMID: 8336752.
- Hernández-Pérez M, et al: Am J Dermatopathol 2012; 34(6): 565-579. doi: 10.1097/DAD. 0b013e31821e8744.
- Muthusamy V, et al: Arch Dermatol Res 2010; 302(1): 5-17. doi: 10.1007/s00403-009-0994-y.
- Tanaka K, et al: Curr Drug Metab 2010; 11(5): 431-435. PMID: 20540695.
- Crisan M, et al: PLoS One 2013; 8(10): e75003. doi: 10.1371/journal.pone.0075003.
- Jariyapamornkoon et al: BMC Complement and Altern Med 2013; 13: 171. doi: 10.1186/1472-6882-13-171.
- Tang JY, et al: J Am Acad Dermatol 2012; 67(5): 803.e1-12, quiz 815-6. doi: 10.1016/j.jaad.2012.05.044.
- Tang JY, et al: J Am Acad Dermatol 2012; 67: 817.e1-11, quiz 827-8. doi: 10.1016/j.jaad.2012.07.022.
- Reid IR, et al: Lancet 2013 Oct 10. pii: S0140-6736(13)61647-5. doi: 10.1016/S0140-6736(13)61647-5.
- Autier P, et al: Vitamin D status and ill health: a systematic review. The Lancet Diabetes & Endocrinology, Available online 6 December 2013. http://dx. doi.org/10.1016/S2213-8587(13)70165-7.
- Peters T: Dermatologist 2011; 62(8): 598-606, doi: 10.1007/s00105-011-2134-9.
- Morita A, et al: J Invest Dermatol Symp Proc 2009; 14: 53-55, doi:10.1038/jidsymp.2009.13.
- Okada HC, et al: Plast Reconstr Surg 2013; 132: 1085-1092, doi: 10.1097/PRS.0b013e3182a4c20a.
- Gilchrest BA: J Invest Dermatol 2013; 133(E1): E2–E6, doi:10.1038/skinbio.2013.176.
- Gordon JRS, et al: N Engl J Med 2012; 366: e25, doi: 10.1056/NEJMicm1104059.
- Valacchi G, et al: Ann NY Acad Sci 2012; 1271: 75-81, doi: 10.1111/j.1749-6632.2012.06724.x.
- Dennerstein L, et al: Menopause Int 2011; 17(3): 96-101. doi: 10.1258/mi.2011.011028.
- Tiesman JP: J Drugs Dermatol 2009; 8(7 Suppl): s12-14. PMID: 19623779.
Dermatology Practice 2014; 24(1): 4-8