Iron is a trace element that is mainly involved in oxygen transport and blood formation in the body. Both iron deficiency and iron excess can severely disrupt the organism and be associated with serious diseases. In particular, the diagnosis of iron deficiency, despite appropriate methods of measurement and the determination of thresholds, proves difficult and poses certain challenges to clinical management.
Nowadays, various parameters can be used to assess iron stores and iron requirements. Bone marrow iron staining is still considered the gold standard for documenting that the body has sufficient iron stores. However, this parameter is no longer regularly used in routine diagnostics, since bone marrow aspiration is generally not part of the clarification of iron deficiency anemia. The diagnostic workup of iron deficiency is therefore usually performed by the serum parameters ferritin, transferrin, and tranferrin saturation, although the parameters ferritin and tansferrin behave differently in the acute phase. In the case of unclear constellations of laboratory values, the determination of the soluble transferrin receptor (sTfR) in the blood can also provide information.
Determination of serum ferritin as a marker of iron supply
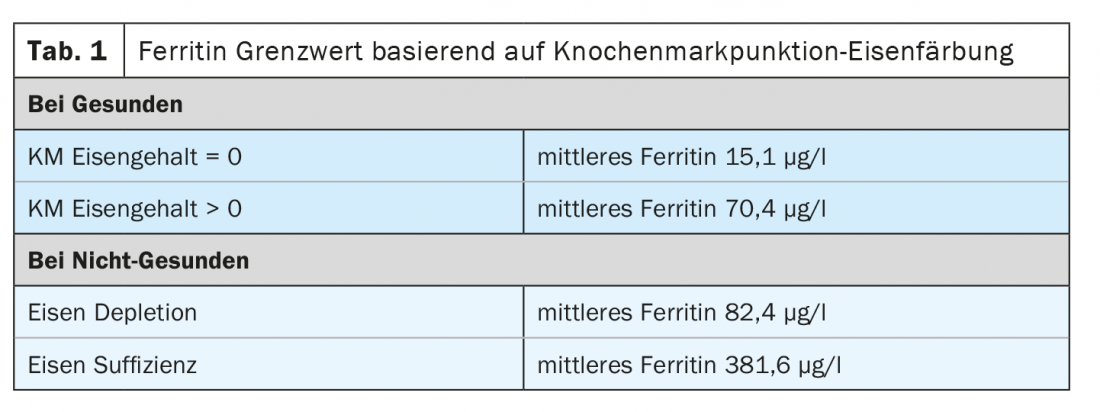
Based on the iron staining of the bone marrow, the ferritin threshold was determined, which provides information on whether iron deficiency is present. However, observational studies have shown that the ferritin threshold varies depending on whether the patient is healthy or not. In healthy patients with positive bone marrow iron levels, the mean ferritin is around 70 µg /l. Patients who do not have positive bone marrow iron but are considered healthy have a mean ferritin of about 15 µg /l. In non-healthy patients, on the other hand, these values shift significantly. In the case of iron depletion, for example, the mean ferritin is around 80 µg /l and in the case of iron sufficiency it is just under 400 µg /l (Table 1) [2].
Thus, the ferritin threshold is subject to fluctuations that may impact accurate measurement and comparability of ferritin concentration. In addition, ferritin assyas from different manufacturers may give different results. For this reason, a World Health Organization (WHO) expert committee has established international reference materials to analyze the performance and comparability of the most common laboratory methods for determining serum or plasma ferritin concentration to detect iron deficiency, saturation, or overload. The results show that the most commonly used laboratory methods for determining ferritin concentration have comparable accuracy and performance. The difference is so small that no relevant statistical significance could be differentiated, so it can be assumed that these do not lead to different clinical management decisions [2].
Challenges in the diagnosis of “iron deficiency
Although knowledge about optimal ferritin values is available and suitable methods for measuring these values are available, it is still difficult to diagnose iron deficiency. Prof. Dr. Med. Wolfgang Korte from the Center for Laboratory Medicine St. Gallen, speaks in this context of a Babylonian confusion [1]. First, different bio-markers are used to define iron deficiency, which all show different performance in the same clinical setting. For example, ferritin will behave dynamically differently than transferrin in the same clinical situation. Second, these bio-markers also perform differently in different clinical settings. Which depends on whether they are used distributionally, in an observational study, or outcome-dependently, in an intervention study. This means that the term “iron deficiency” cannot be defined identically in every situation but must be considered depending on the situation.
As an example, Prof. Dr. Med. Korte cites a study from 2009 in which the shift in ferritin cut-off values in children in an area with high infection pressure was investigated. In this population, two-thirds of the children had malaria. In addition, other bacterial infections and HIV infections were present. The mean ferritin value of the total population was around 700 µg /l. An examination of the various parameters shows that the optimal cut-off of the ROC curve shifts significantly. In contrast to the original cut-off, which in a typical laboratory report is in the range of 30 µg /l, a new cut-off was found in this population in the range of 270 µg /l, an eightfold increase. The results of the study illustrate that the determination of cut-off values cannot be ubiquitous, but population-adapted [3].
Ferritin cut-off values cannot be assessed independently of disease
The fact that appropriate framework and disease conditions must be considered when assessing ferritin cut-off is also demonstrated by intervention studies in which definitions of iron deficiency vary. Chronic heart failure patients, for example, benefit from iron supplementation if iron deficiency is present and defined by a ferritin <100 µg /l or a ferritin <300 µg /l plus transferrin saturation <20%.
A similar situation is seen in renal failure patients who are not dialysis dependent. These patients also benefit from iron supplementation if deficiency is present and defined by a ferritin <0 100 µg /l or a ferritin <200 µg /l plus transferrin saturation <20%, respectively [4,5]. According to of the Health Survey for England, depressive symptoms are also associated with iron deficiency. In this study, the cut-off is far higher at <45 µg /l compared to an intervention study from Zurich, in which non-anemic patients benefited from iron supplementation at a ferritin value ≤15 µg /l [6].
Recommendations for practice
In this respect, “iron deficiency” is not a clearly defined term; rather, the laboratory analytical results must be placed in the clinical setting. At this point, Prof. Dr. Med. Korte refers to the Swiss consensus on the diagnosis and treatment of iron deficiency published in 2019. This states a ferritin threshold of 30 µg /l for the presence of iron deficiency. With ferritin limits between 30 and 50 µg /l, a transferrin saturation <20% may indicate iron deficiency. In principle, the cause of the iron deficiency must be clarified before any treatment. In the case of iron deficiency without anemia, iron supplementation is recommended if appropriate symptoms are present. Supplementation with iron should usually be oral [7].
Take-Home Messages
- “Iron deficiency” is not a clearly defined term.
- The diagnosis of “iron deficiency” is made by different biomarkers in different clinical situations and not by uniform diagnostic criteria.
- The diagnosis requires different thresholds depending on the population or the general condition of the affected person.
- Although detectable differences exist between various ferritin assays, these do not prevent comparable therapeutic decisions.
Literature:
- Prof. Dr. Med. Wolfgang Korte, Iron Deficiency – Laboratory Diagnostics 2021, lecture Iron Academy, 17.06.2021.
- Garcia-Casal, et al: Are Current Serum and Plasma Ferritin Cut-offs for Iron Deficiency and Overload Accurate and Reflecting Iron Status? A Systematic Review. Arch Med Res 2018, doi: 10.1016/j.arcmed.2018.12.005.
- Phiri, et al: New cut-off values for ferritin and soluble transferrin receptor for the assessment of iron deficiency in children in a high infection pressure area. Am J Clin Path 2009, doi: 10.1136/jcp.2009.066498.
- van Veldhuisen, et al: Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Chronic Heart Failure and Iron Deficiency. Circulation 2017, doi: 10.1161/CIRCULATIONAHA.117.027497.
- Macdougall, et al: FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anemia. Nephrol Dial Transplant 2014, doi: 10.1093/ndt/gfu201.
- Krayenbuehl, et al: Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, doi: 10.1182/blood-2011-04-346304.
- Nowak, et al: Swiss Delphi study on iron deficiency. Swiss Med Wkly 2019, doi: 10.4414/smw.2019.20097.
HAUSARZT PRAXIS 2021; 16(11): 48-49
CARDIOVASC 2021; 20(4): 34-35