The efficacy of EGb 761 in cognitive disorders has been documented by a large number of clinical studies. In the broad discussion about the dosage, as well as the sensible start of taking EGb 761 for the prevention and treatment of cognitive disorders, other therapeutic possibilities of EGb 761 are unjustly pushed somewhat into the background. The following article is a summary translated into German of a recently published study on the efficacy of EGb 761 in vertigo [1].
Introduction
Studies show a high prevalence of drowsiness and dizziness. About 17% of all people in Germany seek medical treatment for this at least once in their lives [2]. According to an article published in the German Medical Journal [3], central vestibular vertigo, bilateral peripheral vestibulopathy, and paroxysmal dysfunction of the vestibular nerves are among the common types of vertigo. It often occurs in patients with cerebrovascular disease.
Medications that improve cerebral blood flow are often prescribed to treat vertigo. For the treatment of various types of vertigo, including Meniere’s disease, paroxysmal positional vertigo, other peripheral vertigo, and peripheral vertigo of unknown etiology, betahistine is most commonly prescribed, followed by piroxicam and ginkgo biloba extract [4].
The present study was designed to compare the efficacy and safety of EGb 761 and betahistine in patients with vertigo syndromes.
Study design
This randomized, placebo-controlled, double-blind, multicenter study was conducted in ten different Ukrainian hospitals with 160 outpatients. They had to be at least 45 years old and have had diagnosed unspecified vertigo or unspecified vertigo syndrome for at least three months at recruitment. The strength had to be at least three points on a numerical analog scale (1-10). Furthermore, precise exclusion criteria were defined. After randomization, subjects received either 120 mg of Ginkgo biloba extract EGb 761 twice daily (n = 80) or 16 mg of betahistine twice daily (n = 80) for 12 weeks ( Table 1 ). The demographic data of the two groups showed no significant differences. The two test drugs were administered in such a way that they were indistinguishable in appearance and taste. Daily doses were consistent with efficacy evidence.
Rounds and target variables
In addition to the usual examinations at the beginning of this study, various neuro-otological tests were also made. Efficacy and safety visits were conducted after four, eight, and at the end of the study, i.e., after twelve weeks. Efficacy was measured with a numerical analog scale (NAS) of 0-10, where 0 meant no dizziness and 10 meant extremely severe dizziness. Furthermore, the short version of the Vertigo Symptom Scale (VSS-SF) (Table 2), the Sheehan Disability Scale (SDS) , and the CGI were applied.

Results
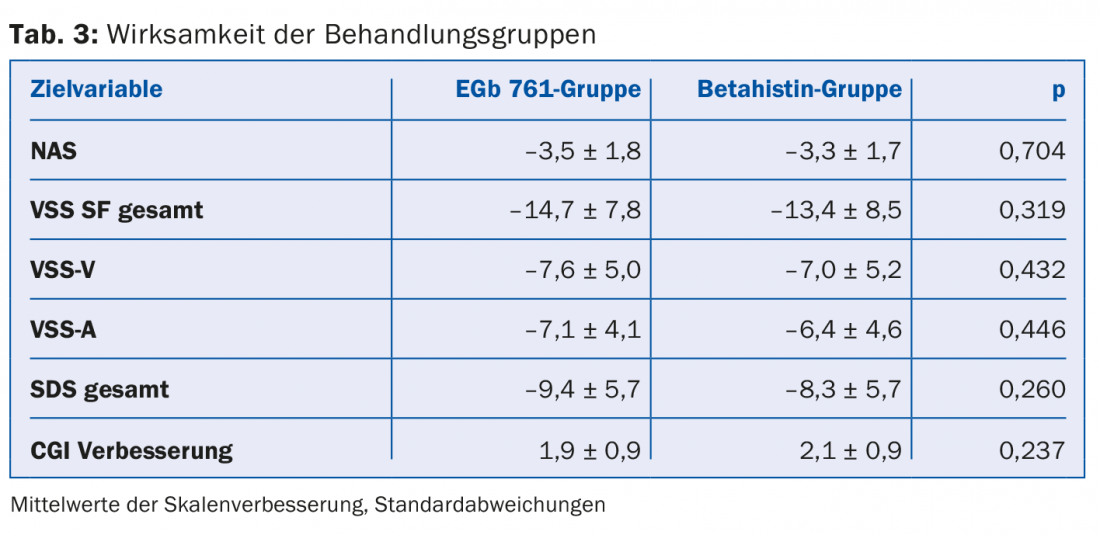
Effectiveness: All drop-outs were accurately justified and documented. The efficacy of the two treatment groups is shown in Table 3.
NAS, VSS-SF, and SDS scores improved markedly in both treatment groups. Similarly, improvement in CGI and neuro-otological scores was seen in both treatment groups. The p-values show no significant difference between the two groups. A subgroup evaluation showed no treatment differences in age, sex, neuro-otologic scores, or symptom severity. In more than 70% of all subjects, the treating physicians rated symptoms as much better or very much better at the end of the study. This evaluation of the physicians and the self-assessment of the patients coincided with the positive results of the neuro-otological examinations.
Safety: of the total 27 reported ADRs in the EGb 761 group (betahistine group 39), none were considered serious. Gastrointestinal disorders, respiratory infections, and headaches were the most common.
Discussion
The present study documents the equivalent beneficial effects of betahistine and EGb 761 for the treatment of patients with nonspecific vertigo. Further, it confirms placebo-controlled studies that showed clinical efficacy of EGb 761 in vestibular and non-vestibular vertigo.
It is noticeable that the improvement in symptoms in the EGb 761 group, although not significant, is clearly evident and numerically greater than in the betahistine group.
Summary
The present study demonstrates the parity of evidence of Ginko biloba extract EGb 761 versus betahistine for the treatment of nonspecific dizziness symptoms.
Literature:
- Sokolova L, Hoerr R, Mishchenko T: Treatment of Vertigo: A Randomized, Double-Blind Trial Comparing Efficacy and Safety of Ginkgo biloba Extract EGb 761 and Betahistine. International Journal of Otolaryngology 2014; 2014: 682439. doi: 10.1155/2014/682439.
- Neuhauser HK, et al: Burden of dizziness and vertigo in the community. Archives of Internal Medicine 2008; 168(19): 2118-2124.
- Strupp M, Brandt T: Leading symptom dizziness: diagnosis and therapy. Deutsches Ärzteblatt International 2008; 105(10): 173-180.
- Okroglic S, et al: Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS ONE 2013; 8(2): e53455. doi: 10.1371/journal.pone.0053455.
HAUSARZT PRAXIS 2016; 11(8): 2-4