SGLT2 inhibitors and GLP1 receptor agonists (GLP1-RA) are antidiabetic drugs with the potential to preserve kidney function. Their different mechanisms of action suggest that combination therapy could have additive or synergistic effects, but this has not yet been tested with albuminuria as the primary endpoint. Danish researchers have investigated the possibility of a combination to prevent the progression of diabetic kidney disease.
Typical complications of type 2 diabetes such as acute myocardial infarction, strokes and amputations have decreased over the past 30 years. This is only true to a lesser extent for end-stage kidney disease (ESKD). This could indicate that there is an unmet need in the treatment of diabetic kidney disease.
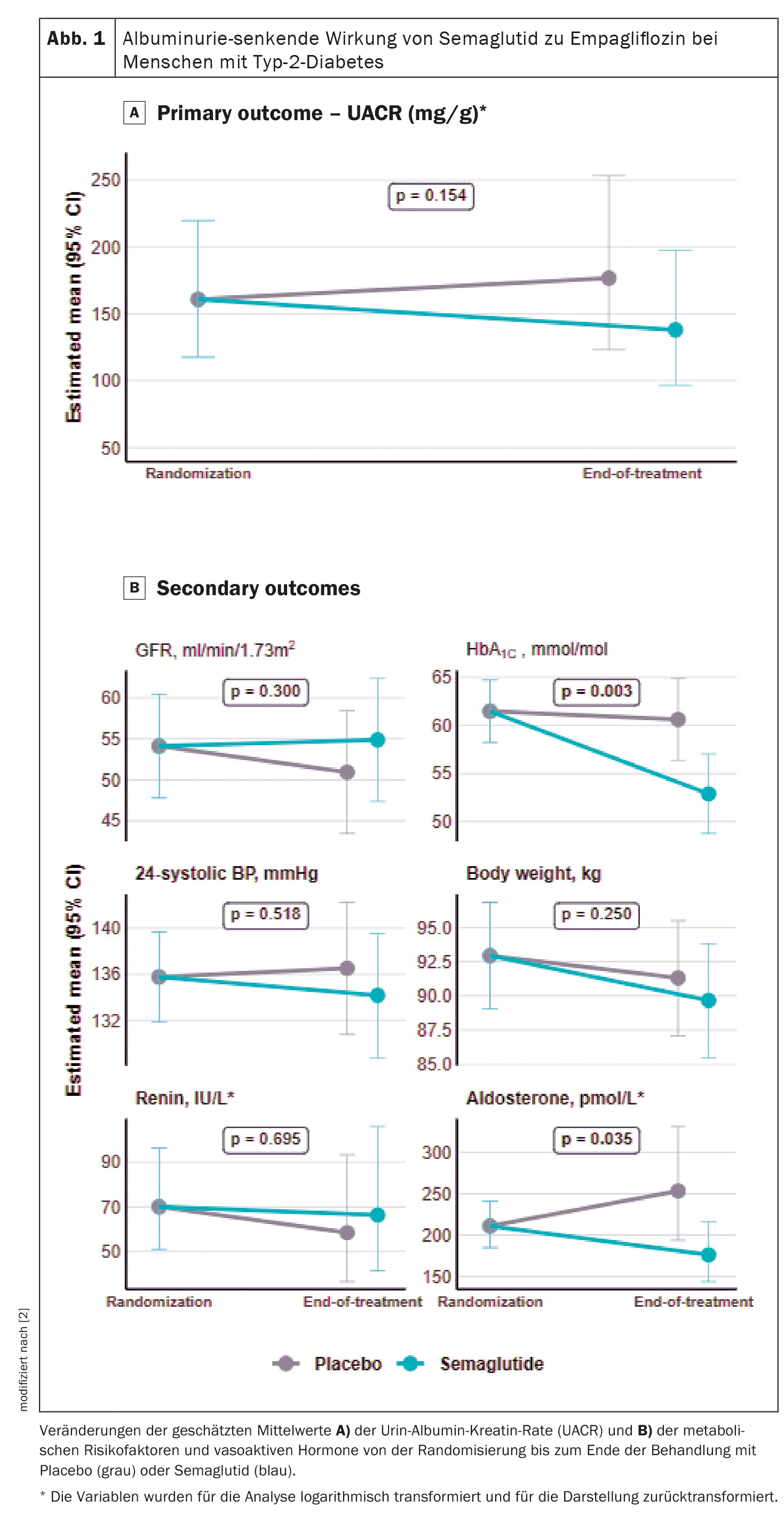
SGLT2 inhibitors are among the most effective means of protecting the kidneys that have been introduced in recent years. DAPA-CKD was one of the studies that firmly anchored SGLT2 inhibitors in the guidelines. Participants who received dapagliflozin showed a greater reduction in albuminuria compared to placebo. However, it also showed that there is still a high residual risk of albuminuria. “Our study aimed to evaluate whether semaglutide (GLP1-RA) vs. placebo added to ongoing empagliflozin (SGLT2) therapy results in a further reduction in urinary albumin-to-creatinine ratio (UACR) in people with type 2 diabetes and albuminuria,” explained Suvanjaa Sivalingam, Steno Diabetes Center, Copenhagen [1]
Empagliflozin plus semaglutide or placebo
The scientists examined 634 participants at the Steno Diabetes Center for their suitability. 73 patients were included in a 26-week run-in phase. All participants received empagliflozin 25 mg once daily. Finally, 60 of the 73 participants were randomized. They were evenly distributed between placebo (n=30) and semaglutide (n=30). At randomization, they were assigned to either semaglutide 1 mg or placebo 1 mg once a week for the remainder of the study period. In the end, the researchers analyzed 26 participants in the placebo group and 28 participants in the semaglutide group.
In addition to obvious type 2 diabetes, the most important inclusion criteria were an eGFR >30 ml/min/1.73m2, albuminuria (UACR) >100 mg/g at inclusion and RAAS blocker treatment at randomization. The primary endpoint was the change in albuminuria (measured in three consecutive urine samples at first morning voiding) from randomization to the end of the study after 26 weeks of treatment. Secondary endpoints included changes in measured GFR(99mTc-DTPA), HbA1c, body weight, 24-hour systolic blood pressure and RAAS hormones.
At the time of randomization, the average age was about 70 years, the majority were men (78%), the average body weight was about 93 kg, the average HbA1c value was 62 mmol/mol, the average systolic blood pressure was 133 mmHg in the placebo group and 138 mmHg in the semaglutide group. The mean GFR was 54 ml/min/1.73m2 and the mean albuminuria at the time of randomization was 148 mg/g in the placebo group and 135 mg/g in the semaglutide group.
Significant reduction in HbA1c, but not in albuminuria
The evaluation of the primary endpoint after 26 weeks of combination treatment showed a numerical reduction in albuminuria with semaglutide of -14% versus 10% in the placebo group with a mean difference of -22% (p=0.154), although this was not statistically significant (Fig. 1A) [2]. In terms of hemodynamic endpoints, the researchers found no changes between the treatment groups in GFR or 24-hour systolic blood pressure (Fig. 1B). “With regard to the metabolic endpoints, the expected reduction in HbA1c-This was also shown in previous studies, but we found no change in body weight between the treatment groups. Nevertheless, there was a numerical reduction of -1.6 kg,” says Sivalingam.
In terms of RAAS hormones, no changes in plasma rennin activity were found between the treatment groups either, “but we found a change in plasma aldosterone activity of -30% between the treatment groups”. However, this result should be interpreted with caution, as in 50% of the participants the measurement of plasma aldosterone activity was below the detection limit and therefore not all participants could be included in the analysis.
The speaker qualified that there were limitations in the study, including recruitment problems due to the COVID-19 pandemic. In addition, the inclusion of participants in the study was based on the historical albuminuria value and not on the actual albuminuria value. “Accordingly, the albuminuria level was not confirmed at randomization, which means that there could be participants who were uncertain whether their albuminuria level had changed or even normalized during the run-in phase.”
Since there are few other studies that have examined the same target but similar endpoints, the researchers plan to collect the data and perform a meta-analysis to see if this increases the power and changes the results. “We believe that a certain group of people will benefit from this combination, especially if they have ischemic heart disease, uncontrolled diabetes or are obese.” However, larger studies and further research are needed to evaluate this.
Take-Home-Messages
- Combination therapy with empagliflozin and semaglutide did not lead to a reduction in albuminuria in participants with type 2 diabetes and albuminuria compared to empagliflozin alone.
- However, the study confirmed a significant reduction in the HbA1c value.
- The measured GFR, body weight and 24-hour systolic blood pressure did not change.
- Semaglutide reduced aldosterone activity in plasma, while renin activity in plasma did not change.
Congress: EASD 2023
Sources:
- Sivalingam S: Presentation “Renal effects of empagliflozin alone or in combination with semaglutide in albuminuric type 2 diabetes: a randomized, placebo-controlled trial”; EASD Congress 2023, Hamburg, 5.10.2023.
- Sivalingam S, Soendergaard Wasehuus V, Rotbain Curovic V, et al: Albuminuria-lowering effect of adding semaglutide on top of empagliflozin in individuals with type 2 diabetes: A randomized and placebo-controlled study. Diabetes Obes Metab 2024; 26(1): 54-64; doi: 10.1111/dom.15287.
InFo DIABETOLOGY & ENDOCRINOLOGY 2024; 1(1): 22-24 (published on 14.2.24, ahead of print)