Aortic valve stenosis is the most common valve disease in the Western population. Surgical correction by reconstruction has been used almost exclusively for aortic valve regurgitation. With the TriRec procedure, an option is now available to reconstruct the aortic valve from autologous pericardium even in the presence of stenosis.
With a frequency of 2% within the population over 65 years of age, aortic valve stenosis (AS) represents the most common valve disease in the Western population. It accounts for 1.3% in people aged 65 to 75 and 4% in those aged 85 and older. The most common cause of stenosis is valve degeneration [1]. Two percent of the population congenitally have a bicuspid aortic valve. In these patients, valve degeneration occurs at a younger age because of the different biomechanical forces acting on the valve and aorta due to the bicuspid configuration of the valve [2].
In advanced aortic valve disease, surgical aortic valve replacement (ACE) is the treatment of choice. In patients who have reached the age of 85 and in patients with significant concomitant disease, catheter-based aortic valve implantation has become increasingly established as an alternative treatment procedure. Biological or mechanical valve prostheses are available for surgical valve replacement, and both types of valves are associated with a number of specific advantages and disadvantages. In cases of pure aortic valve insufficiency, the valve can also be reconstructed. However, the majority of patients develop aortic valve stenosis. For these, no successful and convincing reconstruction procedure existed so far.
Trileaflet Aortic Valve Reconstruction (TriRec) with autologous pericardium
In 2011, Prof. S. Ozaki (Tokyo, Japan) described a highly standardized surgical procedure for the aortic valve that can always be used regardless of the underlying pathology. In this procedure, three neo-aortic pockets are made from the patient’s own pericardium. After implantation of the new leaflets, a fully functional and tricuspid aortic valve is created [3].
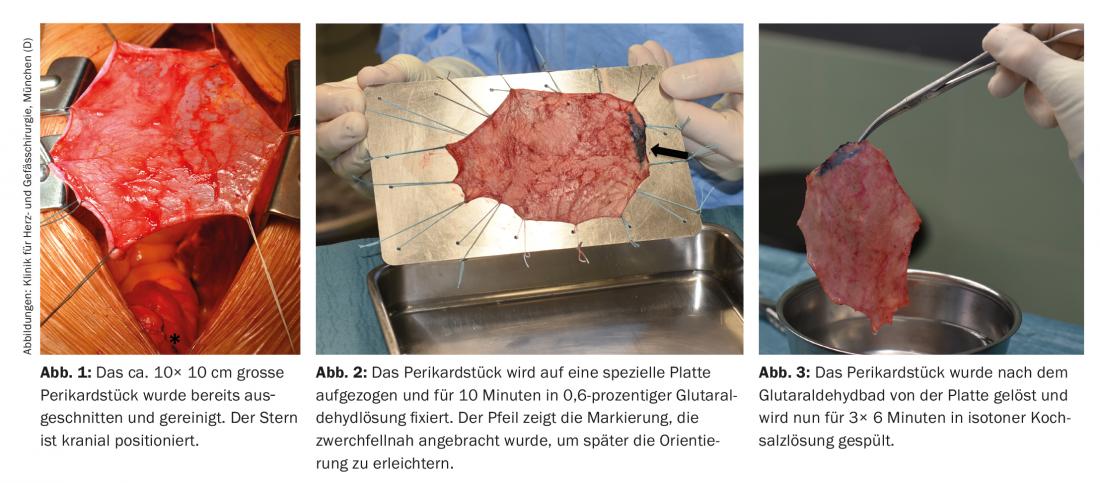
Access for this operation is always via a complete median sternotomy. After opening the sternum, the first step is to prepare the pericardium: already in situ, it is freed from fat and connective tissue (Fig. 1) . Subsequently, a piece measuring approx. 10× 10 cm is harvested. The pericardium is placed in 0.6% glutaraldehyde for ten minutes and then rinsed in isotonic saline for 3× 6 minutes (Figs. 2 and 3).
In the next step, the patient is connected to the heart-lung machine and cardioplegic arrest is induced in mild hypothermia. The ascending aorta is opened 1.5 cm above the right coronary ostium with a transverse incision. The leaflets of the diseased aortic valve are removed, and any calcifications in the valve annulus must be removed very carefully. Subsequently, the size of the neo-aortic pockets is determined with a special “sizer” (Fig. 4).

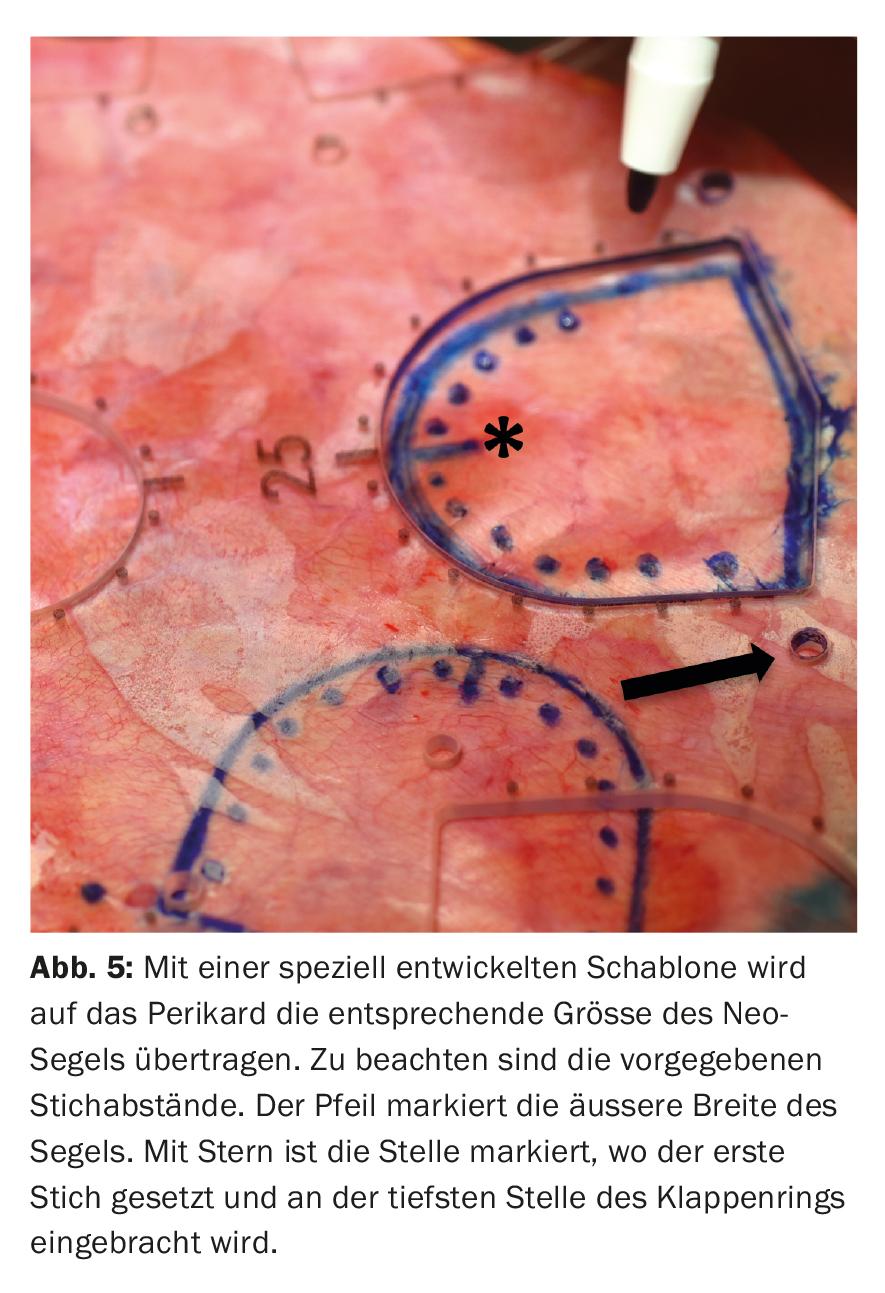
Special care must be taken to ensure correct placement of the “sizer” between the respective commissures to accurately determine the size of the new sail. If a bicuspid valve was initially present, tricuspidization of the aortic valve is always performed at our hospital. Analogous to the determined size, the respective sail is now cut from the prepared pericardium using templates. It should be noted that a tissue margin of 5 mm is left in the area that will later form the commissures. In addition, the templates specify stitch distances that are transferred to the neo sails (Fig. 5).
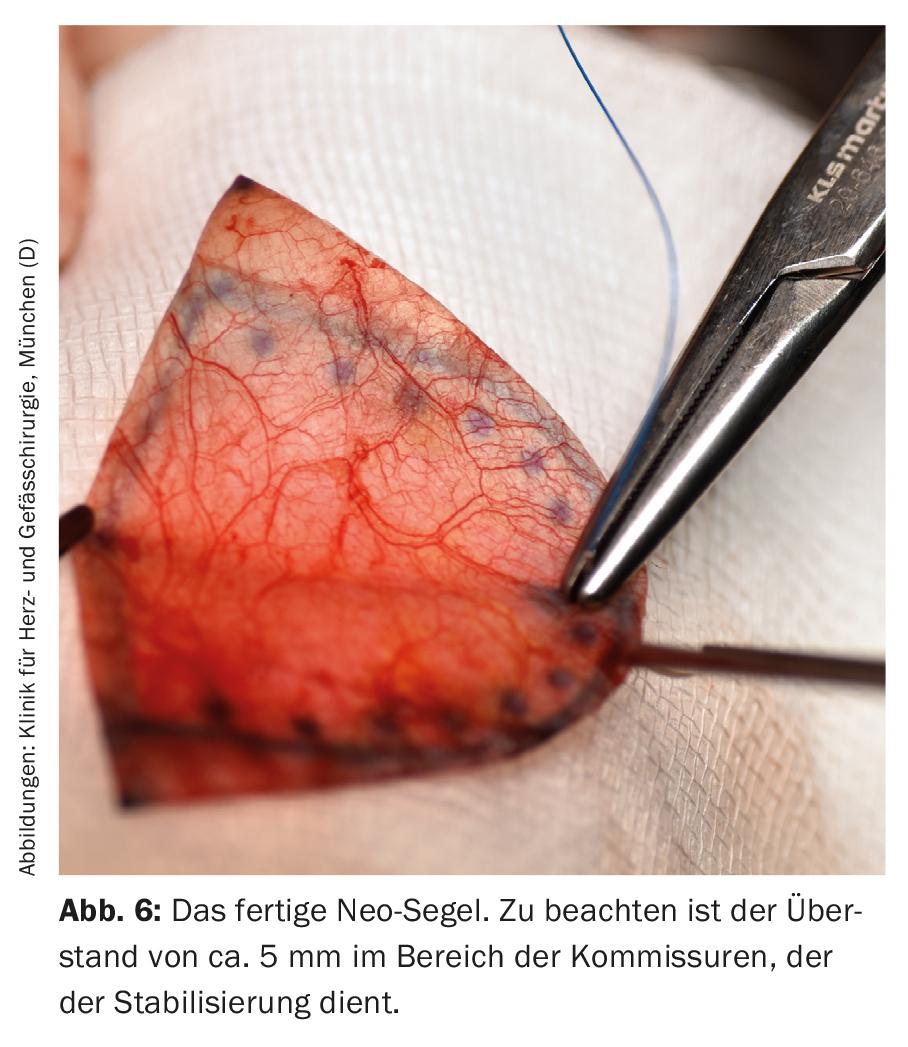
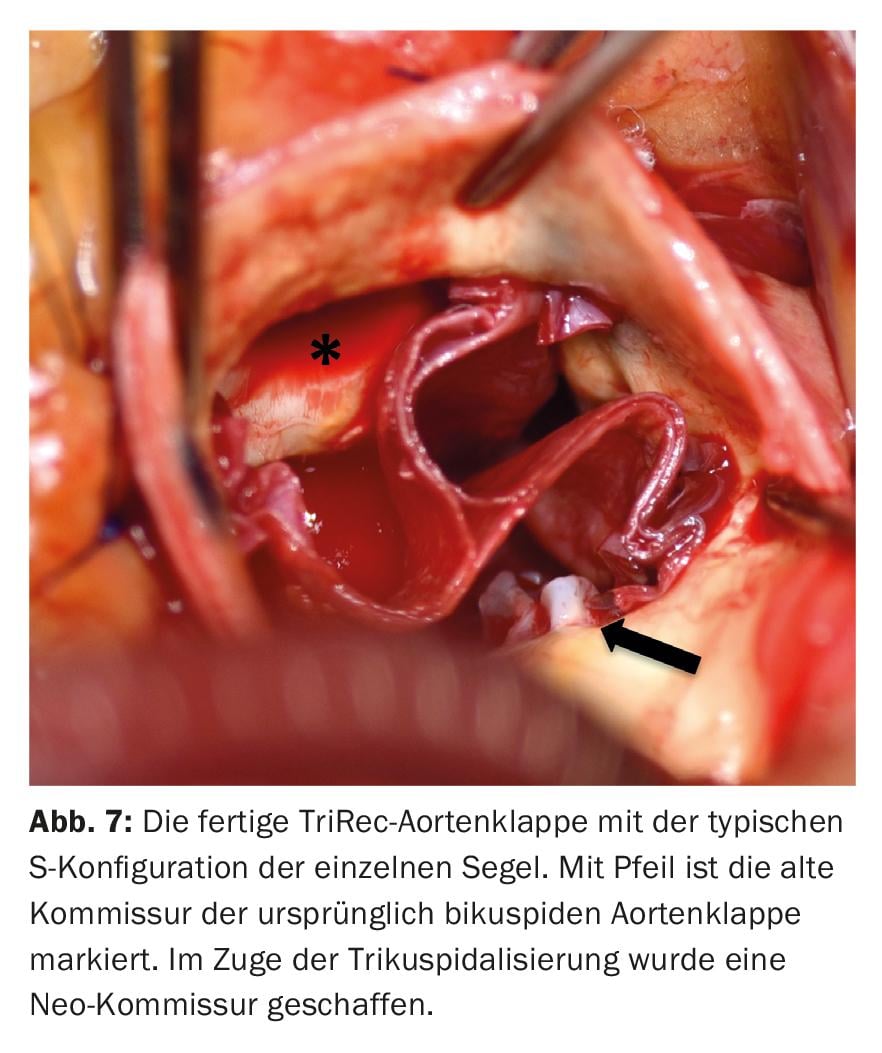
The new sails are attached to the native annulus with a continuous suture, starting at the lowest point in each case and maintaining the pre-designed distances between the individual stitches (Fig. 6). Once the height of the associated commissure is reached, the threads are stitched outwards and knotted over felt pledgets. In the commissure area, the seams are reinforced with additional Teflon-armored seams. Before the aortotomy is closed, the new valve is evaluated under direct vision and by water sampling (Fig. 7) . After weaning from the heart-lung machine, the new valve is evaluated on the beating heart with transesophageal echocardiography with regard to tightness, gradients and valve opening area.
Discussion
Surgical aortic valve replacement has been performed with either a biological or mechanical valve prosthesis. Both types of valves have specific advantages and disadvantages: mechanical prostheses theoretically have an unlimited lifetime, but patients must undergo lifelong anticoagulation treatment with the risk of thrombosis, prosthesis malfunction, or bleeding. In contrast, biological prostheses do not require permanent anticoagulation. However, the disadvantage of this type of prosthesis is its limited service life. Because biological valve prostheses also degenerate by calcification, reoperations are unavoidable, especially in younger patients [4]. Common disadvantages of both types of valves are the introduction of foreign material into the human body and the fixation of the natural annulus of the aortic valve by the suture ring of the prosthesis. This leads to an abrogation of the physiological dynamics of the natural aortic valve annulus.
Valve reconstruction techniques can almost only be used in aortic valve regurgitation, where the leaflets are not calcified and are still mobile [5]. Until now, there has been no successful and convincing concept to avoid implantation of a prosthesis in patients with aortic valve stenosis and severe calcifications and correspondingly limited leaflet mobility.
This is the great advantage of the TriRec process. The native, decalcified aortic annulus, which guarantees the physiological mobility of the valve apparatus during the cardiac cycle, is left in place during the reconstruction procedure and is not fixed by a rigid ring of a prosthesis. The near-physiological mobility of the new pericardial sails combined with a large coaptation surface reduces the mechanical stress acting on the sails. Another potential benefit of the TriRec procedure may be that a larger catheter valve can be implanted into the then native annulus if a follow-up procedure is required. Especially with small biological prostheses (19 or 21 mm), one is currently severely limited in the choice of a catheter valve prosthesis due to the stiff prosthetic ring.
The data published to date by Prof. S. Ozaki and colleagues show stable long-term results for a period of up to 70 months after reconstruction in a collective ranging from children to over 80-year-old patients. Over the observation period, freedom from reoperation was shown to be excellent at 96.7%. The peak gradient across the reconstructed aortic valve was less than 20 mmHg [6].
Summary and outlook
The treatment of aortic valve stenosis in the vast majority of cases today is surgical replacement of the valve with an artificial prosthesis. However, this procedure also has disadvantages, such as an increased risk of endocarditis due to foreign material, degeneration of the prosthesis if a biological one is used, or the need to take oral anticoagulants if the prosthesis type is mechanical (Table 1).
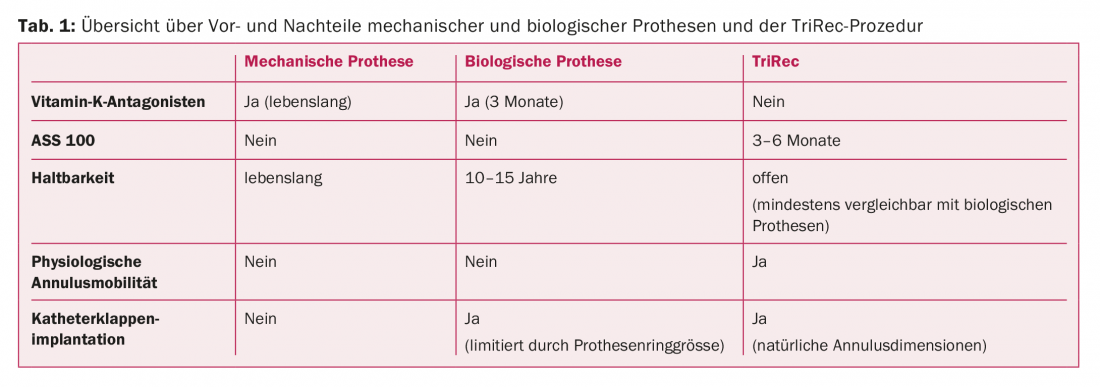
An alternative to the implantation of a valve prosthesis is the TriRec procedure. In addition to the use of autologous tissue, the procedure offers further advantages for patients: the natural dynamics of the aortic valve annulus remain unaffected during the procedure, and the use of anticoagulants can also be dispensed with with regard to the valve. Data from over 850 patients operated on in this manner in Japan show excellent long-term results in terms of durability of valve function associated with low hemodynamic gradients and low risk of endocarditis [7].
Take-Home Messages
- Aortic valve stenosis is the most common valve disease in the Western population.
- Surgical correction by reconstruction has been used almost exclusively for aortic valve regurgitation.
- The TriRec procedure now provides an option to reconstruct the aortic valve from autologous pericardium even in the presence of stenosis.
- The disadvantages of previous valve replacement procedures (biological or mechanical), such as the insertion of foreign material and fixation of the annulus by the suture ring of the prosthesis, are eliminated.
- The use of anticoagulants can be dispensed with in the TriRec procedure.
Link to info film: http://dhm.mhn.de/de/kliniken_und_institute/klinik_fuer_herz-_und_gefaessc/patienteninformation/ozaki_operationsmethode.cfm
Literature:
- Otto CM, et al: Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999; 341(3): 142-147.
- Sievers HH, Schmidtke C: A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5): 1226-1233.
- Ozaki S, et al: Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interact Cardiovasc Thorac Surg 2011; 12(4): 550-553.
- Goldstone AB, et al: Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N Engl J Med 2017; 377(19): 1847-1857.
- Boodhwani M, El Khoury G: Aortic valve repair. Oper Tech Thorac Cardiovasc Surg 2010; 14(4): 266-280.
- Ozaki S, et al: Aortic Valve Reconstruction Using Autologous Pericardium for Aortic Stenosis. Circ J 2015; 79(7): 1504-1510.
- Ozaki S: Mid-term outcomes in 850 patients treated with aortic valve neo-cuspidization using glutaraldehyde-treated autologous pericardium. (https://aats.blob.core.windows.net/media/17AM/2017-05-03/RM302-304/05-03-17_Room302-304_0742_Ozaki.mp4)
CARDIOVASC 2018; 17(1): 28-31











