Malignant melanoma of the skin has a high metastatic rate and accounts for more than 90% of all skin cancer-related deaths. Therefore, early detection and best possible treatment is very important. The European Association of Dermatooncology (EADO), together with the European Dermatology Forum (EDF) and with the European Organization for Research and Treatment of Cancer (EORTC), has issued new guidelines for the diagnosis and treatment of melanoma in 2022.
At the Annual Meeting of the European Association of Dermato-Oncology (EADO), Prof. Dr. med. Claus Garbe, University Dermatology Clinic Tübingen, gave an update on important aspects of the guideline [1,2]. Regarding screening and diagnostics, the acting EADO president stated that for the evaluation of pigmented and non-pigmented skin lesions, dermoscopy is still considered the standard. For high-risk patients, the guideline additionally recommends the use of whole-body photography to detect melanoma as early as possible. Digital dermoscopy can also improve early detection of melanoma and should be used in high-risk patients with a high total number of nevi. About 70% of melanomas are newly formed and 30% develop from melanocytic nevi, Prof. Garbe explained. Using modern technologies such as “Body Scan”, new lesions can be reliably identified. This automatically compares the current full-body images with those from the last examination, so that both changed and newly added nevi are detected. When there is a clinical suspicion of melanoma, histopathologic confirmation is mandatory. Four main types of malignant melanoma can be distinguished according to the growth pattern and localization: superficial spreading melanoma is the most common, followed by nodular melanoma, lentigo maligna, and acrolentiginous melanoma [3].
Ultrasound of locoregional lymph nodes recommended
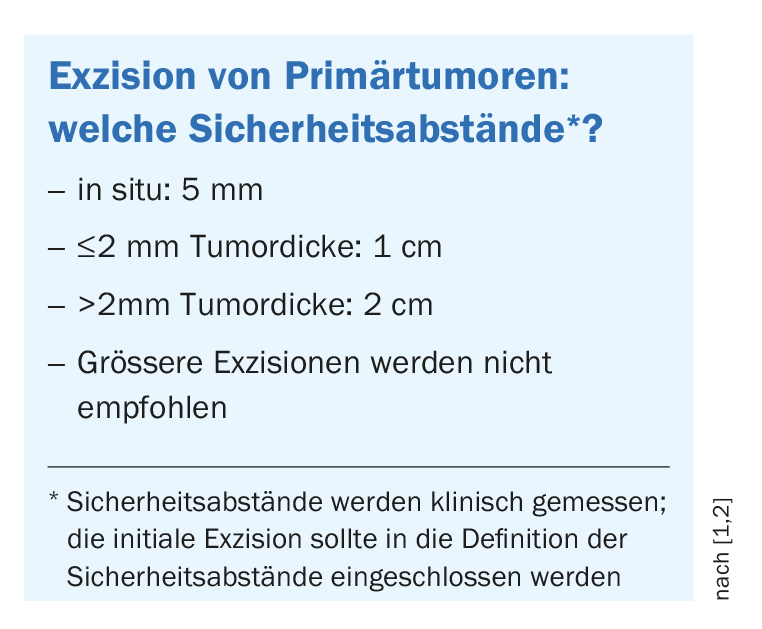
For staging of primary melanoma pT1b**, ultrasound diagnosis of locoregional lymph nodes and the in-transit pathway is recommended at both initial workup and follow-up. If melanoma is clinically suspected, it should be completely excised immediately with a small safety margin (1-3 mm) (box ). Incisional biopsies are useful for large lesions such as those on the face (e.g., lentigo maligna), for acral lesions, or for lesions in the genital area. If histologic examination shows that it is malignant melanoma, further re-excision with 1-2 cm safety distance should be performed [4] to prevent the likelihood of local recurrence. For some melanoma subtypes, such as lentigo maligna or genital and acral melanomas, microscopically controlled surgery may be used to spare surrounding tissue and ensure complete resection. In Germany, this is used relatively often, the speaker reported. For example, the “Slow Mohs” technique is used.
** pT = histopathologically assessed primary tumor
Sentinel lymph node biopsy: much-discussed topic
For a correct tumor classification (staging) and as a basis for the therapy decision, patients with a tumor thickness ≥1.0 mm (or in case of additional histological risk factors from ≥0.8 mm) should be offered a sentinel lymph node biopsy (SLNB) [1,2]. “This will be a source of debate in the future,” Prof. Garbe said, adding that it is uncertain whether SLNB will still be performed in a few years [1]. Originally, SLNB served the purpose of indicating complete lymphadenectomy. But recent studies show that survival is not improved in patients with complete lymphadenectomy, the EADO president reported [1]. According to the current guideline, complete lymphadenectomy should no longer be performed in patients with micrometastases in the sentinel lymph node. It is an indication for adjuvant systemic therapy. Complete resection or other destructive procedures may be considered for oligo-metastasis. Metastasectomy was recommended in the past, but nowadays treatment using immune checkpoint inhibitors or targeted therapy is favored. Radiotherapy of the primary tumor is rarely indicated in melanoma. But for patients who refuse surgical treatment, for example, radiotherapy could be considered, he said.
Better outcomes thanks to modern drug therapy regimens
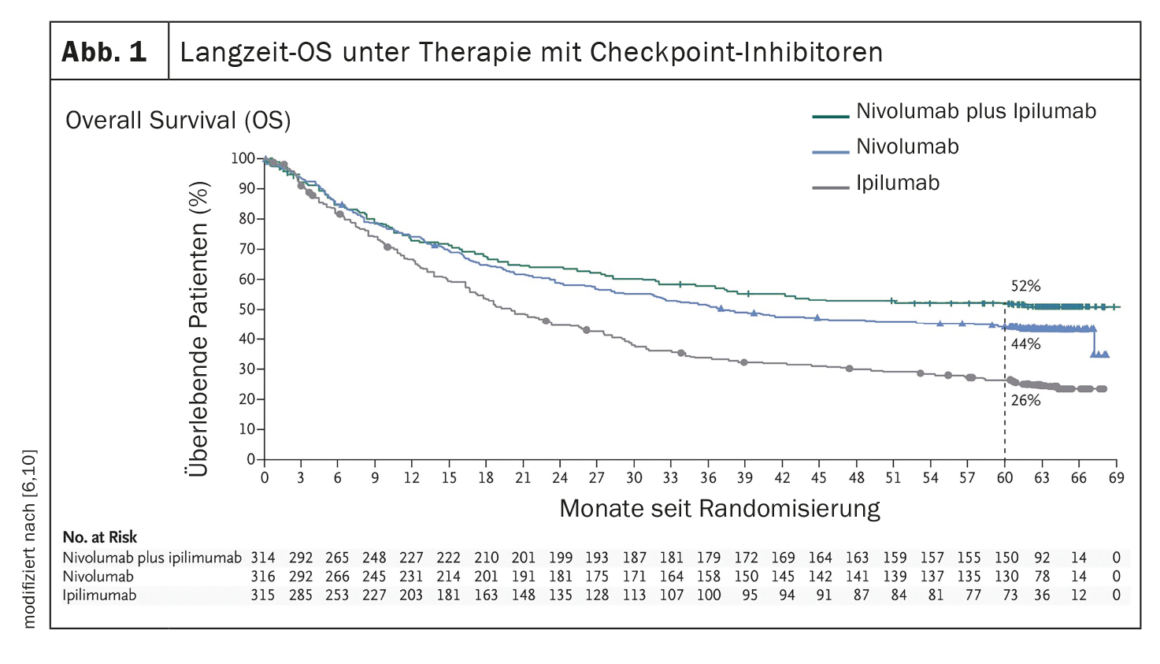
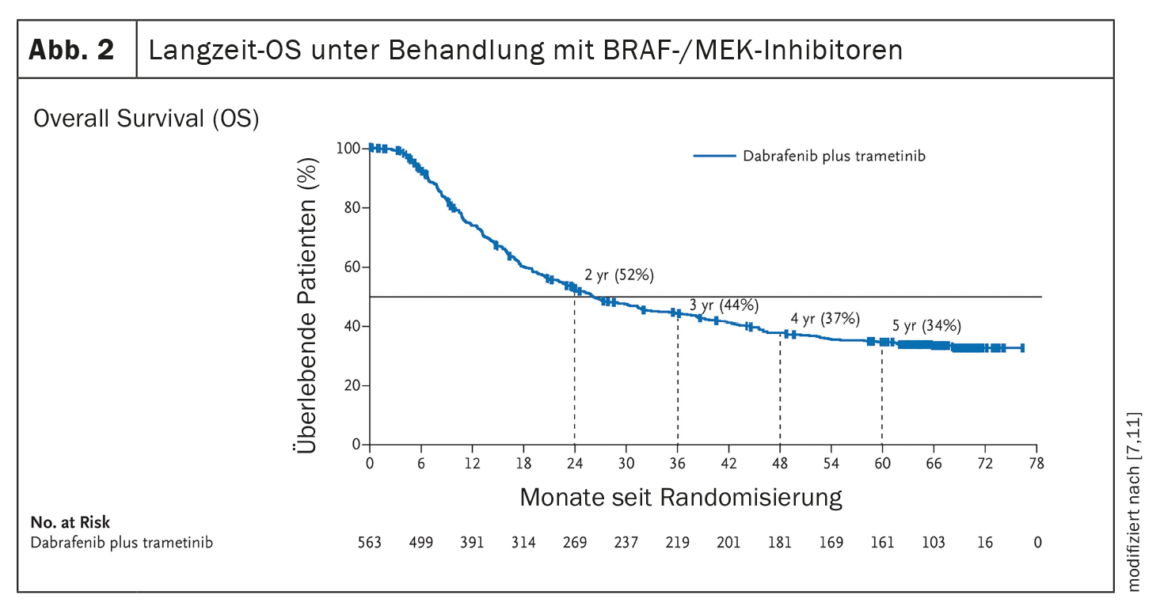
The establishment of systems therapies such as immune checkpoint inhibition or targeted therapy with BRAF/MEK inhibition (in the presence of a BRAF V600 mutation) have led to a significant improvement in the clinical course of patients with metastatic melanoma (Fig. 1, Fig. 2). In the era of chemotherapy, the median survival of patients with unresectable metastases was 7 months and the 3-year survival was 5%, according to data published in 2008 [5]. In contrast, data from the CheckMate067 trial published in 2019 showed a 5-year overall survival (OS) of 52% in the treatment arm with nivolumab plus ipilimumab and 44% with nivolumab monotherapy, respectively, when treated with immune checkpoint inhibitors (Fig. 1) [6]. And treatment with the BRAF inhibitor dabrafenib (150 mg twice daily) plus the MEK inhibitor trametinib (2 mg once daily) achieved 5-year OS rates of 34% (95% CI; 30-38) [7] (Fig. 2 ).
Immunotherapy or targeted therapy as first-line?
Based on current data, immune checkpoint inhibition should be offered as first-line treatment in stage IV patients, even regardless of BRAF mutation status. Combined treatment with nivolumab and ipilimumab is favored over monotherapy, Prof. Garbe explained. In stage IV melanoma, determination of PD-L1 expression is not required and should not be a criterion for treatment decisions. In certain situations, patients with stage IV melanoma and proven BRAFV600E or V600K mutation may be offered first-line BRAF/MEK inhibitor therapy as an alternative to immunotherapy. There are data showing that certain patients benefit when targeted therapy is used right at the beginning.
Therapy in the adjuvant setting
Concepts of adjuvant systemic therapy to prevent distant metastases, prolong survival, and increase long-term survival have been tested for many years. Adjuvant therapy can reduce the risk of recurrence in patients with resected malignant melanoma and should be offered to all patients in stage IIIA-IIID [1,2]. Anti PD-1 therapy is an option regardless of mutation status, while adjuvant BRAF/MEK inhibitor therapy may also be considered for patients with BRAFV600/K mutation. For adjuvant treatment of stage IV melanoma (after complete resection), the guideline advises suggesting nivolumab as a treatment option to patients regardless of mutation status. “These adjuvant therapies are amazingly effective,” Prof. Garbe emphasized, saying that he is not currently aware of any other tumor entities with comparably effective adjuvant therapy options. That a recurrence-free survival (RFS) of 70% was achieved at 24 months with the combination of nivolumab and ipilumab in patients with stage IV NED melanoma (“no evidence of disease”; no residuals after resection), compared with 42% with nivolumab monotherapy and 14% with placebo, underscores the efficacy of checkpoint inhibition in the adjuvant setting [1,8]. However, adjuvant treatment using BRAF/MEK inhibition has also provided convincing evidence of efficacy i. Dabrafenib plus trametinib reduced the risk of tumor recurrence or death (RFS) by 53% compared with placebo after a median follow-up of 2.8 years (HR 0.47; 95% CI; 0.39-0.58; p<0.001) [1,9,13].
Congress: EADO Annual Meeting
Literature:
- «Recommendations from the updated European Interdisciplinary Melanoma Guideline 2022 (EADO/EORTC)», Prof. Dr. med. Claus Garbe, Symposium 4, EADO Annual Meeting, 20.04.2023.
- Garbe C, et al.; EDF, EADO, and EORTC. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment – Update 2022. Eur J Cancer 2022; 170: 256–284.
- Innovationsreport 2021, www.tk.de/resource, (letzter Abruf 22.05.2023)
- Läuchli S: Chirurgische Techniken: Dermatochirurgische Möglichkeiten für die Behandlung von Hauttumoren in der Praxis. Dermatologie Praxis 2023; 2: 12–15.
- Korn EL, et al.: Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008; 26(4): 527–534.
- Larkin J, et al.: Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2019; 381(16): 1535–1546.
- Robert C, et al.: Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med 2019; 381(7): 626–663.
- Schadendorf D, et al.: ESMO 2019 Oral BA67.
- Hauschild A: Abstract LBA6_PR, ESMO 2017.
- Larkin J, et al.: Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2019; 381(16): 1535–1546.
- Robert C, et al.: Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med 2019; 381(7): 626–636
- Diaz-Ramón JL, et al.: Cancers 2023; 15: 2174. www.mdpi.com/2072-6694/15/7/2174#, (letzter Abruf 23.05.2023)
- ESMO 2017: Adjuvant Dabrafenib Plus Trametinib Significantly Lowers Risk of Death in Stage III BRAF V600–Mutated Melanoma, www.esmo.org/oncology-news/archive; (letzter Abruf 23.05.2023)
DERMATOLOGIE PRAXIS 2023; 33(3): 26–27