Adjuvant systemic treatment improves survival and freedom from recurrence in early breast cancer. Genetic testing of the tumor can help in the choice of therapy. Supportive monitoring is useful even years after completion of adjuvant therapy.
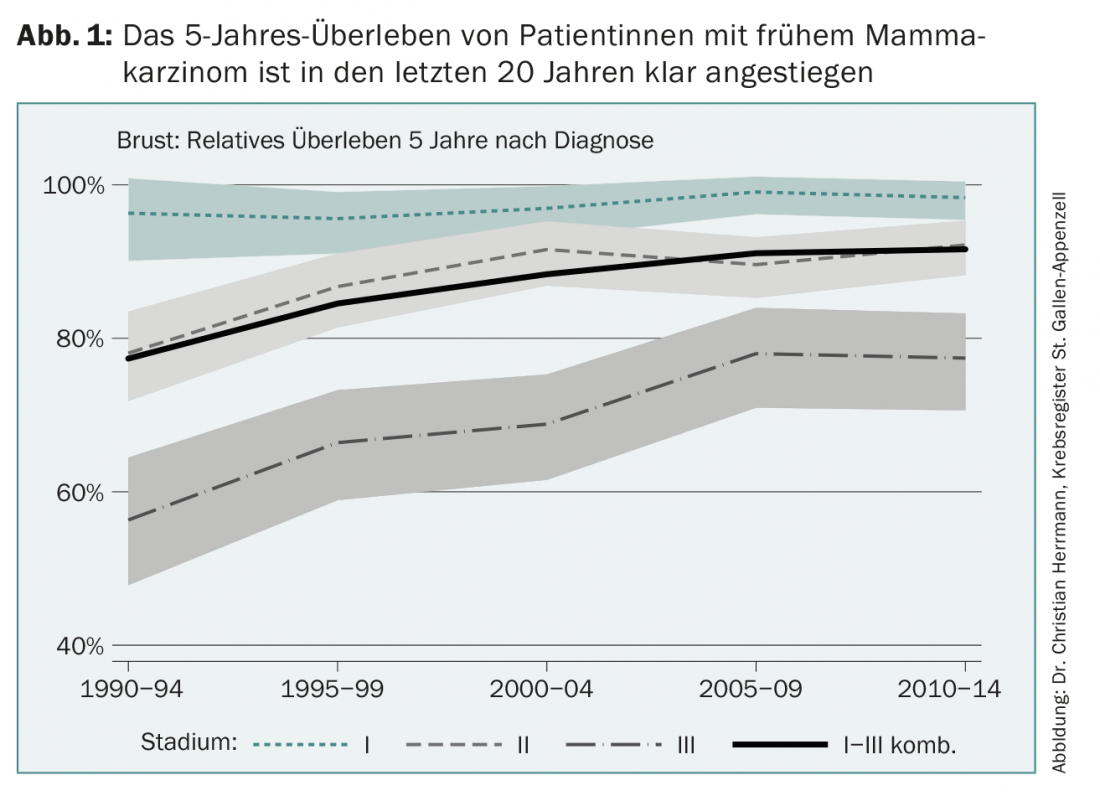
Adjuvant therapy for early breast cancer has made great strides since its inception just over 40 years ago. Today, more than 80% of women with breast cancer in Europe can be cured (Fig. 1). This progress has several causes, such as improved early detection, mammography screening programs, new surgical and radiation options, but also the continuous development of the additional drug system therapies called adjuvant. This success story is the result of international randomized trials and their subsequent translation into clinical practice, a process that is by no means self-evident. In some Asian countries, for example, longer-term survival after newly detected breast cancer is now only about 40% for a variety of reasons.
The regular St. Gallen conferences on the treatment of early breast carcinoma, now held at two-year intervals, provide a good illustration of scientific developments since the 1980s. These congresses are each accompanied by a consensus of internationally renowned breast cancer experts, which receives worldwide attention and is prominently published.
In the interdisciplinary discussions in the regular tumor boards, recognized guidelines are translated into individualized optimized therapy planning, a procedure institutionalized in the breast centers that contributes significantly to the improved therapy results.
The 15th St. Gallen Congress on the topic of primary therapy of early breast carcinoma was held for the second time in Vienna from March 15 to 18, 2017 and was held under the motto of individualized therapy planning. This should be as non-invasive as possible, but as strong as necessary (“de-escalating and escalating treatments for early stage breast cancer”). The subsequently formulated consensus recommendations can be found in the Annals of Oncology [1]. In earlier years, the personalization of therapy in adaptation to the different biology of tumors had been pointed out. General recommendations should additionally be adapted to the individual value standards, wishes and possibilities of the patients concerned, as well as to the economic possibilities. The panel also notes in the consensus that randomized trials cannot provide adequate answers in all individual cases and to all relevant clinical questions.
With the increased long-term survival of breast cancer patients (“survivors”), other aspects are gaining importance, e.g. long-term consequences of therapies and social issues such as professional reintegration.
Whether and which additional system therapy is possible and useful depends primarily on the biology of the carcinoma, but also on the staging, and thus on the likelihood of recurrence or metastasis.
Different types of breast carcinomas are distinguished on the basis of molecular biology, a classification that can, however, be made in a similar manner in practice according to previous consensus recommendations using widely available immunohistochemical methods [2]. These subtypes are referred to as Luminal A, Luminal B, HER 2-positive, and tripel-negative and exhibit different behavior with corresponding treatment recommendations.
Hormonally based therapies
In the case of immunohistochemically detected estrogen receptors in tumor cells, adjuvant hormone therapy is recommended, in the case of luminal A tumors often as sole systemic therapy or also in addition to chemotherapy, depending on the constellation. Low expression of estrogen receptors is also sufficient for this indication.
In postmenopausal women, one of the aromatase inhibitors letrozole, anastrozole, or exemestane is preferable to tamoxifen because of slightly better efficacy. In case of tolerance problems or comorbidities, tamoxifen can be used as an alternative due to the different side effect profile, especially if the risk of recurrence is lower.
Clinical trials showed that ten years of adjuvant hormonal therapy, whether with tamoxifen [3], an aromatase inhibitor [4], or a sequence, is somewhat superior to a previously standard five-year treatment period. This longer duration of therapy achieves clinical significance especially in women with an increased risk of relapse.
In premenopausal women, tamoxifen monotherapy remains an accepted good treatment option. Additional LHRH agonist therapy or an aromatase inhibitor and LHRH agonist are slightly more effective, as randomized trials have shown, but are associated with considerably more severe side effects [5]. This is generally justified in cases of higher risk of recurrence, such as in women under 35 years of age, tumors with a poorer degree of differentiation, and involvement of at least four axillary lymph nodes; situations that have also usually led to the choice of additional adjuvant chemotherapy.
Chemotherapy for receptor-positive breast carcinoma.
Additional adjuvant chemotherapy for receptor-positive tumors is indicated when there is a higher risk of recurrence; in general, this is already the case for Luminal B type.
The risk of recurrence can be inferred from biology and is also dependent on tumor stage, particularly the number of axillary lymph nodes affected. Important factors are the degree of differentiation and the proliferation rate, which can be measured immunohistochemically with the marker Ki-67. Standardization and reproducibility of this quantitative factor was a challenge for pathologists.
An interesting additional prognostic parameter is the degree of infiltration of the tumor by lymphocytes. A higher number of TILs (tumor infiltrating lymphocytes) indicates a more pronounced antitumor immune response and is associated with a better prognosis. However, this factor is currently insufficiently standardized to be used for therapeutic decisions in routine practice.
There are also internet-based ways to obtain an indication of prognosis with an algorithm based on clinical/pathologic data, which can be an aid in counseling patients (www.adjuvantonline.com or Predict www.predict.nhs.uk). The latter algorithm also incorporates the Ki-67 proliferation level and HER 2 status.
Validated and reproducible genetic testing on tumor material may further influence the decision for or against additional adjuvant chemotherapy. This is especially true in receptor-positive breast carcinomas with no or limited axillary lymph node involvement, when the test result indicates a very good prognosis and therefore allows additional chemotherapy to be avoided. There are several established competing tests available to clinicians, such as Oncotype®, Endopredict® or Mammaprint®. The use of such tests in these situations is explicitly supported by the St. Gallen Consensus.
HER 2-positive tumors
One year of treatment with trastuzumab, which is currently also available in subcutaneous form, in addition to chemotherapy and possibly anti-hormonal therapy substantially improves recurrence-free survival. Chemotherapy may be anthracycline- and taxane-containing therapy; in small tumors with lower risk of recurrence, it may be limited to taxanes alone.
In the preoperative situation, combined administration of the anti-HER-2 antibodies trastuzumab and pertuzumab can additionally substantially improve response [6]. This also suggested adjuvant postoperative use. The highly anticipated data from the Aphinity trials were presented at the 2017 ASCO Congress and showed a statistically significant improvement in freedom from recurrence, but in absolute terms in a small range of 1% [7]. Therefore, in the adjuvant setting, this expensive additional treatment is likely to provide a clinically relevant additional benefit only in a restricted group of patients at increased risk of relapse.
Triple-negative carcinomas
These tumors lacking expression of estrogen and progesterone receptors and lacking overexpression of HER 2 receptors are biologically a heterogeneous group. If adjuvant therapy is chosen, only chemotherapy can be considered, usually a combination of anthracyclines/alkylants and taxanes.
Breast carcinomas with BRCA 1 or 2 mutations are clustered, but by no means always triple-negative carcinomas. In these patients, platinum-containing chemotherapy may be chosen. Outside of currently ongoing trials, the use of PARP inhibitors in this situation is not (yet?) justified.
Neoadjuvant therapies
While there is no survival benefit when necessary systemic therapy is given prior to surgery for breast cancer (i.e., neoadjuvant). However, the operation must then only cover the residual tumor area, i.e. not the original tumor extension. If a sentinel node scan performed after chemotherapy shows no tumor involvement, axillary excision can be avoided. The advantage of neoadjuvant treatment is therefore the possibility of less invasive surgical therapy, which may even be breast-conserving despite an initially large tumor.
Neoadjuvant therapy also allows time for surgical planning, which may be important in women with suspected BRCA mutations to undergo genetic counseling and genetic analysis and then discuss the option of mastectomy, possibly even bilaterally.
The consensus recommends neoadjuvant therapies for HER 2-positive as well as tripel-negative breast carcinomas.
Antiresorptive therapies
Based on the Austrian studies that zoledronate or denosumab not only protect against osteoporosis but also reduce the risk of relapse [8,9], the consensus panel accepted that additional treatment with bisphosphonates (not yet denosumab) was indicated in postmenopausal hormonal status.
Supportive therapies
Many patients are very receptive to dietary recommendations. To be able to contribute to recovery in an area that is generally the subject of much debate and to retain control over treatment, at least in part, is motivating. Although there is no actual diet that patients hope will have an effect on an existing tumor, nutritional counseling is useful. Both chemotherapy and (anti)hormonal treatments may occasionally cause marked weight gain. Proactive counseling is therefore helpful for this reason, including balanced information that a sugar-, lactose- or gluten-free diet is an unproven measure in cancer treatment. However, obesity has a proven efficacy in the development of cancer and patients with breast cancer without obesity can hope for a lower mortality [10] and recurrence rate.
An additional important aspect is sufficient physical exercise. There is increasing data that physical activity can reduce recurrence rates [11]. Appropriate orientation and references to regional offers are to be made.
In 2010, there were already almost 300,000 so-called “survivors” of various tumor types in Switzerland, of which breast cancer represents the most significant group [12]. Due to the continuously increasing numbers, today there will be about 350,000. There are special programs, according to the Krebsliga Ostschweiz (Cancer League of Eastern Switzerland), that counsel women in these areas after they have completed tumor treatment. Another important service is psycho-oncological support. In addition, it is important to actively prevent osteoporosis, especially with aromatase inhibitors (bone density determination, sufficient calcium and vitamin D intake, if necessary also Prolia®).
After intensive adjuvant treatment, a considerable number of patients suffer from fatigue, which often lasts for a long time, as well as reduced concentration, difficulty finding words and forgetfulness (“chemo brain”). These complaints should not simply be dismissed as psychogenic. There is evidence that points to organic, measurable changes in the brain and has shown inflammatory processes in the brain to be causal [13].
Outlook
Other new drugs will find their way into adjuvant therapy, possibly cyclin-dependent kinase 4 and 6 inhibitors (palbociclib) and PARP inhibitors in patients with BRCA mutations. Treatment of patients with neratinib following trastuzumab in HER 2-positive carcinomas yielded a lower recurrence rate [14]. One potential hope in triple-negative breast carcinomas is the new immunotherapies.
With modern neoadjuvant therapies for HER 2-positive breast carcinoma, a complete response with no residual tumor can be seen at surgery in more than half of situations. Whether surgery can be avoided entirely in such situations will be the subject of clinical trials. In this case, drug therapy would no longer be adjuvant, but would be the main therapy for early breast carcinoma.
Take-Home Messages
- Adjuvant systemic treatment markedly improves survival and freedom from recurrence in early breast cancer.
- An interdisciplinary treatment team with regular tumor board is an important factor for optimal therapy recommendation.
- Genetic testing of the tumor may help in the choice of adjuvant therapy or lead to omission of chemotherapy.
- Supportive care even years after completion of adjuvant therapy is an important part of treatment (“survivorship program”).
Literature:
- Curigliano G, et al: De-escalating and Escalating Treatments for Early Stage Breast Cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Annals of Oncology 2017; 28(8): 1700-1712.
- Goldhirsch A, et al: Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22(8): 1736-1747.
- Davies C, et al: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381(9869): 805-816.
- Goss PE, et al: Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med 2016; 375(3): 209-219.
- Pagani O, et al: Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371: 107-118.
- Gianni L, et al: 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016; 17(6): 791-800.
- Von Minckwitz G, et al: Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017; 377: 122-131.
- Gnant M, et al: Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozole plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 2015; 26: 313-320.
- Gnant M, et al: Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 433-443.
- Chan DS, et al: Body mass index and survival in women with breast cancer – systematic literature review and meta-analysis of 82 follow-up studies. Annals Oncology 2014; 25(10): 1901-1914.
- Dieli-Conwright CM: Reducing the Risk of Breast Cancer Recurrence: an Evaluation of the Effects and Mechanisms of Diet and Exercise. Curr Breast Cancer Rep 2016; 8(3): 139-150.
- Herrmann C: Cancer survivors in Switzerland: a rapidly growing population to care for. BMC Cancer 2013; 13: 287.
- Morant R: Chemotherapy-associated cognitive impairment. Info @ oncology 2016; 6: 14-17.
- Chan A, et al: Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, pl acebo-controlled, phase 3 trial. Lancet Oncology 2016; 17: 367-377.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(4): 10-13.