Brachytherapy (BT) is a form of radiotherapy in which a radioactive source is precisely placed in the tumor or tumor bed for usually a short period of time. It is indicated for a broad spectrum of tumor entities. The high-precision form of irradiation is limited to the region at risk and allows a high dose to be delivered while sparing the surrounding tissue.
Brachytherapy (BT) is a form of radiotherapy in which a radioactive source is precisely placed in the tumor or tumor bed for usually a short period of time. It is indicated for a wide range of tumor entities [1]. The focally high radiation dose with simultaneous deep exposure of the surrounding healthy tissue explains the treatment successes. The first part of the review article will explain general physical and practical aspects of BT. The second part will highlight the evidence-based use of BT for the main indications and the respective treatment process.
Terminology
The prefix “brachy” comes from the Greek and means “short,” as in the short-range radiation that characterizes this form of treatment. In French-speaking countries, the term “Curiethérapie” is used, named after Marie and Pierre Curie, who laid important foundations of BT with the discovery of radium and the proposal around 1901 to introduce a radiation source into a tumor. BT should be distinguished from radionuclide therapies used in nuclear medicine (i.e., lutetium-PSMA therapy, for example); unlike these therapies, radio-oncology uses closed sources (rather than open sources) and applies them in a locally controlled manner (rather than systemically).
Irradiation “from the inside vs. from the outside” – BT vs. EBRT
Schematically, two forms of dose application can be distinguished in radio-oncology; percutaneous irradiation (external-beam radiotherapy, EBRT) and BT. In EBRT techniques (such as intensity-modulated radiotherapy, stereotactic irradiation, or proton therapy), the therapy beam is generated outside the patient, and the irradiation device can be switched on and off. In BT, a radioactive source measuring a few millimeters is introduced into the tumor or tumor bed, usually for only a short time. The source is continuously active here, can be extended and retracted but not switched on or off. The radiation intensity decreases over time according to the natural decay of the selected radionuclide (in the case of iridium-192, half of the original activity is still present after about 2.5 months). In order to keep the treatment time consistently low, the clinic for Iridium-192, for example. a source change made every 3-4 months.
In radiotherapy, uncertainties of positioning or due to intrinsic organ movements are compensated with an additional safety margin, typically measuring 3-5 millimeters, around the desired treatment volume (so-called “PTV margin”). Since the applicator or irradiation source moves with the tumor (bed) during BT, this safety margin is omitted – the irradiation volume is therefore smaller. The influence of the safety margin on the total volume can be well illustrated by the frequently cited example of an orange [2]. If you take away an orange’s peel of a few millimeters, its volume is halved (the sphere’s volume shrinks with the third power of the radius). In BT, the safety margin (i.e., the “orange peel”) can be omitted, thus limiting the irradiation volume to the actual risk region.
Forms of BT
Depending on the type of application chosen, the instruments used or the dose rate, BT can be classified differently. With respect to the location of the tumor tissue to be covered with the applicator, a distinction is made between contact brachytherapy, intracavitary BT, and interstitial BT:
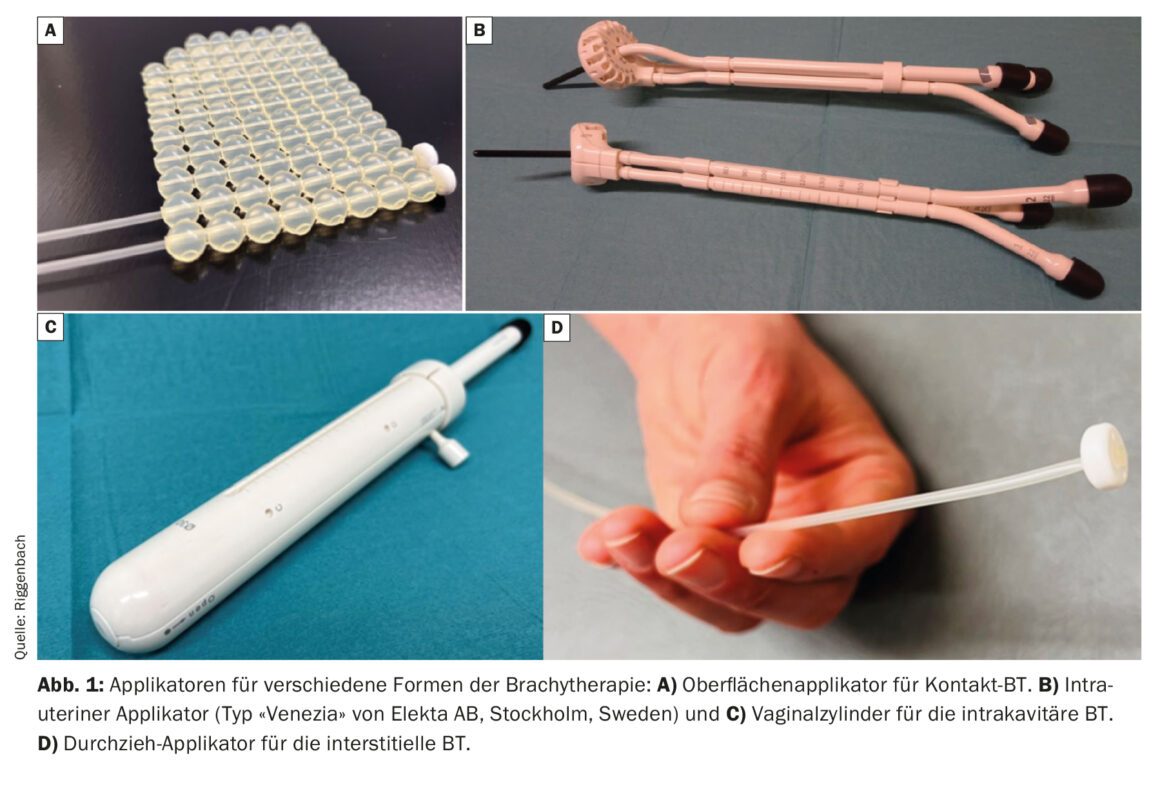
Contact brachytherapy allows custom-made moulages to be attached to the skin. Catheters are integrated into these moulages, which are spaced approximately 1 cm apart and approximately 5 mm from the skin [3]. A special form of contact therapy is intraoperative irradiation; here, the surgical site from which the tumor was previously resected can be treated as the still microscopic tumor-bearing surface (Fig. 1) [4,5].
Intracavitary BT involves bringing the radiation source close to the target volume via natural body cavities. Common indications are tumors of the female genital tract (insertion of a vaginal cylinder for the treatment of vaginal dome in operated endometrial carcinoma or intrauterine applicator insertion in definitive radiotherapy of cervical carcinoma) [6,7]. For BT of elongated cavities (esophagus [8], bronchus, nasopharynx or rectum), there are special applicators in different diameters and lengths in each case.
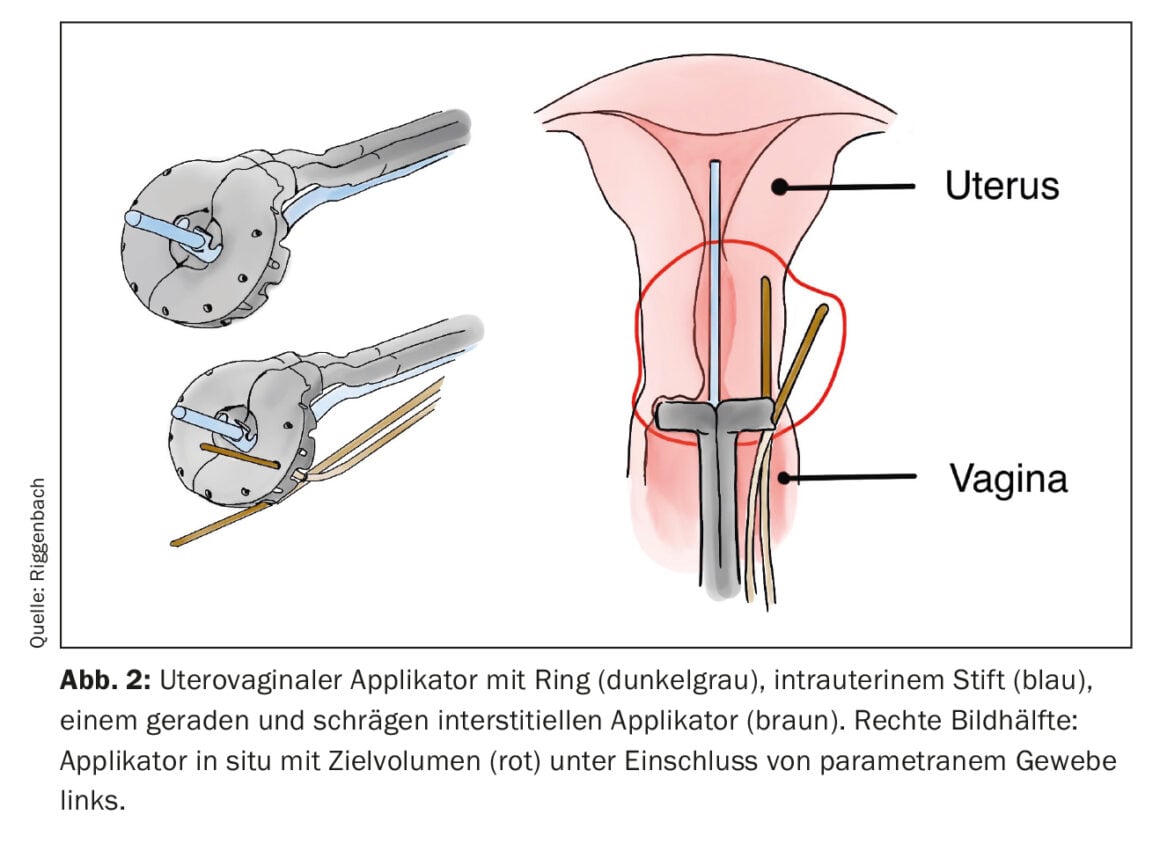
If the target region to be treated is further away from the skin or mucosa, or the tumor exceeds a certain thickness, interstitial BT is more appropriate. For this purpose, flexible plastic tubes are inserted directly into the tumor or tumor bed via metal or rigid plastic guides (so-called cribbing). Common examples of the use of interstitial BT include partial breast irradiation or additional treatment of the parametria via interstitial needles in cervical cancer. With a parallel and equidistant arrangement of the interstitially inserted applicators, a more homogeneous dose distribution is possible. For the regular arrangement, lancing can be supported via industrially manufactured hole arrangements (e.g. on the ring surface of uterovaginal applicators) (Fig. 2) . Alternatively, the insertion is image-guided using the freehand technique.
Classified according to dose rate (number of grays per time), continuous long-term irradiation (LDR, low-dose rate BT) can be distinguished from fractionated short-term irradiation (HDR, high-dose rate BT). It determines the radionuclide selected. In LDR-BT, which is essentially still used in prostate treatment, iodine-125 is usually used as the radioactive source. Encapsulated iodine-125 seeds are permanently placed interstitially.
In temporary HDR-BT, the most commonly used emitter is Iridium-192. Due to the high dose rate, the source has already deposited the desired dose in the tumor(/bed) after a short time and is removed right after this. In order for the insertion and extraction to be accurate to the second and millimeter, an applicator, needle or catheter must first be placed, through whose cavity the source can be passed in the afterloading procedure.
A special form of HDR BT is PDR (pulsed dose rate) BT, in which an HDR source is typically used to deliver a therapy pulse every hour via, for example, a pulse generator. one week of treatment is applied in the inpatient setting. Although tumor biologically favorable, PDR is increasingly being replaced by HDR-BT internationally for logistical reasons and is not currently offered in any center in Switzerland either.
Physical principles
The physical reason why the radiation used in BT is called short-range is due to the quadratic distance law – if the distance from the source is doubled, the dose is reduced to a quarter. Because in BT the source is immediately adjacent to the area to be irradiated (i.e., the distance is small), this law is much more significant than in percutaneous irradiation at the linear accelerator (where the distance of the tumor to the “source” or accelerator head is typically about one meter). When the distance is increased from 1 cm to 2 cm and 3 cm, the dose decreases by 75% and 90%, respectively, with BT, whereas it is reduced by only a few percent with percutaneous therapy. Of course, in the case of percutaneous irradiation, many other factors such as radiation attenuation by tissue would have to be taken into account to explain the actual dose distribution. In BT, on the other hand, the distance-squared law accounts for the largest dose contribution, especially near the source. The dose therefore decreases very rapidly with BT, the so-called dose gradient is steep, which on the one hand leads to very high dose peaks in the immediate vicinity of the source or the tumor and on the other hand exposes surrounding risk organs only slightly.
Practical procedure of HDR brachytherapy
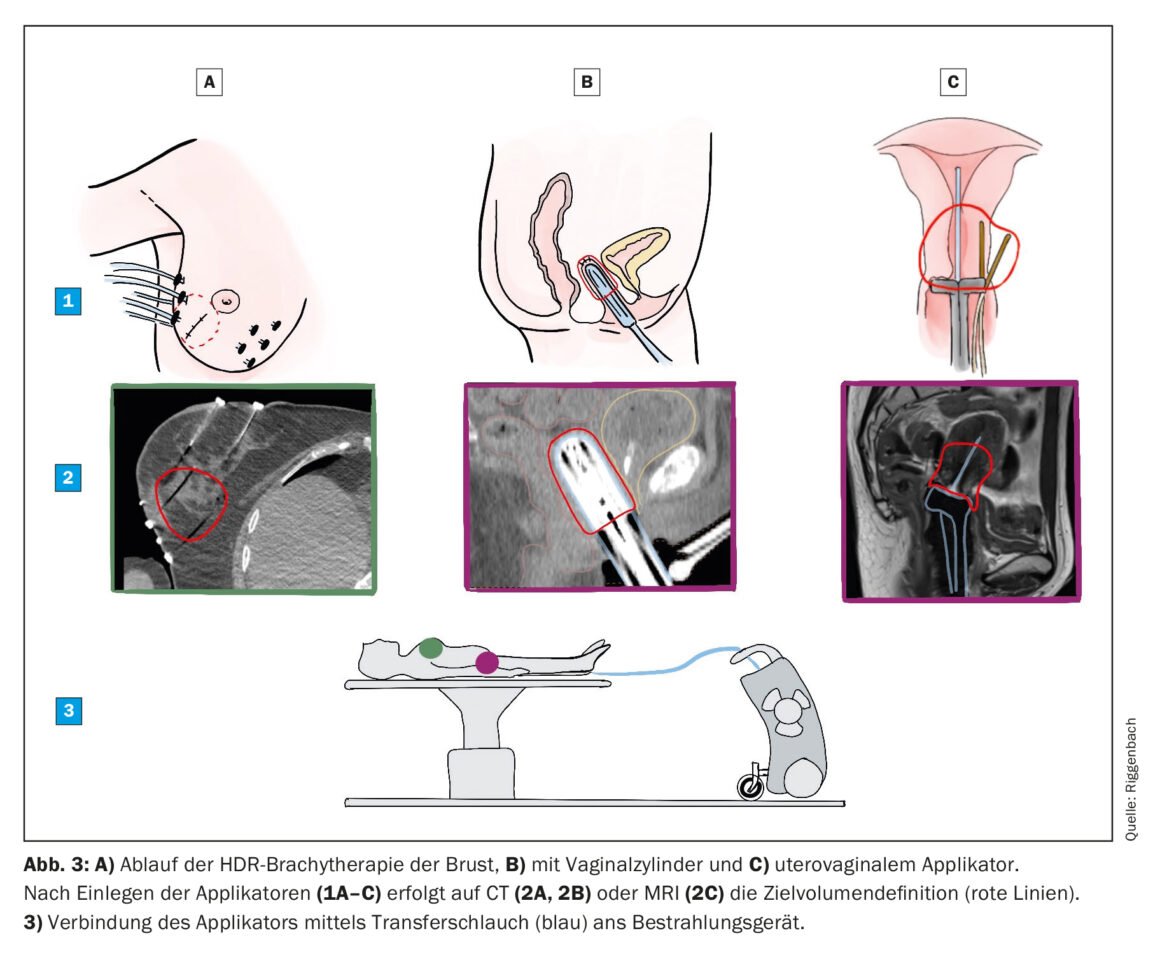
Most BT treatments today are performed using the reload method for logistical and radiation protection reasons. For this purpose, an inactive applicator is first inserted without radiation. Each applicator has a cavity through which the radiation source is passed in a later step. The Iridum-192 source is about the size of a grain of rice and is attached to the end of a thin wire stored in a mobile safe. After applicator insertion, a CT and/or an MRI is performed on which the target volume and organs at risk are marked. According to this, the medical physicist is responsible for the preparation of the irradiation plan. The source position (within the length of the applicator) and the irradiation time at the respective position can be freely selected and is optimized by the planning software. Once the targets have been reached, the irradiation plan is sent to the irradiation console, the patient is escorted to the treatment room and the applicator placed inside is connected to the safe (Fig. 3). The treatment is started and controlled remotely from the adjacent room. It takes only a few minutes, after which the radiation source is automatically retracted back into the safe and the applicator is removed.
Challenges and outlook
The complexity of brachytherapy is not based in the technique, but in the manual skills of insertion and the interaction of the professional staff within the radio-oncology and interdisciplinary. It is labor-intensive, but its simple technology means that it remains cost-efficient, and this is one of the reasons why it is so important in resource-poor countries. It is one of the oldest treatment options in oncology, with >100 years of experience, and as a result is sometimes considered historical or outdated. Today’s brachytherapy with modern applicators, image-guided insertion and software-based dose optimization has implemented medical progress and fits perfectly into the approaches of “individualized medicine”. Like all modalities of radiotherapies, it is organ-preserving, but its minimally invasive approach, modern image implementation, and broad use require a dedicated, interdisciplinary team of experts.
Take-Home Messages
- Brachytherapy is a highly precise form of treatment in which
the irradiation volume is limited to the actual risk region. - The steep dose gradient results in a high dose in the target region while sparing the surrounding tissue as much as possible.
- It can be applied on the surface (contact BT), in a body cavity (intracavitary BT), or in tissue (interstitial BT).
- The most common use is temporary HDR-BT, in which a high dose of
is applied in a few sessions using the reloading method. - Its broad use makes brachytherapy an interdisciplinary
Form of treatment.
Literature:
- Chargari C, Deutsch E, Blanchard P, et al: Brachytherapy: An overview for clinicians. CA Cancer J Clin 2019; 69(5): 386-401.
- Verellen D, Ridder M De, Linthout N, et al: Innovations in image-guided radiotherapy. Nat Rev Cancer 2007;7(12): 949-960.
- Guinot JL, Rembielak A, Perez-Calatayud J, et al: GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother Oncol 2018; 126(3): 377-385.
- Roeder F, Krempien R: Intraoperative radiation therapy (IORT) in soft-tissue sarcoma. Radiat Oncol 2017.
- Tom MC, Joshi N, Vicini F, et al: The American Brachytherapy Society consensus statement on intraoperative radiation therapy. Brachytherapy 2019;18(3): 242-257.
- Harkenrider MM, Block AM, Alektiar KM, et al: American Brachytherapy Task Group Report: Adjuvant vaginal brachytherapy for early-stage endometrial cancer: A comprehensive review. Brachytherapy 2017; 16(1): 95-108.
- Schmid MP, Fokdal L, Westerveld H, et al: Recommendations from gynaecological (GYN) GEC-ESTRO working group – ACROP: Target concept for image guided adaptive brachytherapy in primary vaginal cancer. Radiother Oncol 2020;145: 36-44.
- Rovirosa Á, Tagliaferri L, Chicheł A, et al: Why is a very easy, useful, old technique underused? An overview of esophageal brachytherapy-interventional radiotherapy. J Contemp Brachytherapy 2022;14(3): 299-309.
InFo ONCOLOGY & HEMATOLOGY 2023; 11(2): 6-9.