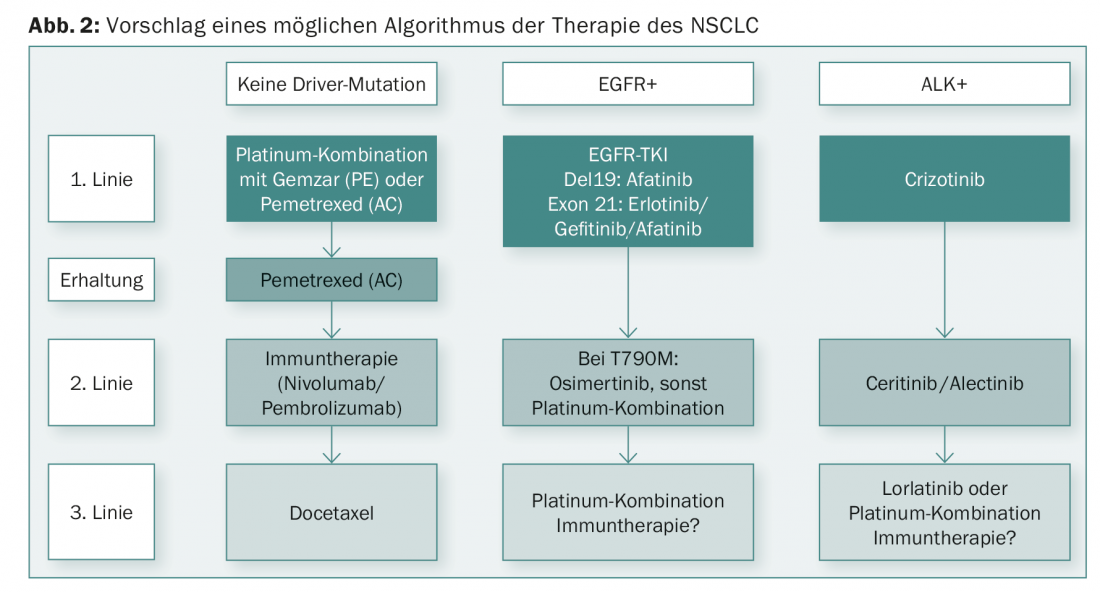
Detailed molecular diagnosis is now mandatory for non-small cell lung cancer (NSCLC). In the presence of a driver mutation, the corresponding tyrosine kinase inhibitor should be used in the first line if possible. In case of progression of these tumors, rebiopsy is very important to identify specifically targetable resistance mechanisms. For NSCLC without a driver mutation, cytotoxic chemotherapy with a platinum is still the therapy of choice as first-line treatment. In second-line therapy, immunotherapy is clearly superior to previous treatments and may be considered the new standard.
No other tumor claims nearly as many lives worldwide as bronchus carcinoma [1]. Smoking is the triggering factor in more than 80% of all patients. In Switzerland, the incidence in men has been decreasing slightly in recent decades (currently 51/100,000/year), but unfortunately a steep increase has been observed in women due to smoking behavior (27/100,000/year) [2]. The incidence of small cell lung cancer has been steadily decreasing for the past 20 years and still accounts for 15% of all lung cancers. The term non-small cell carcinoma (NSCLC) subsumes adenocarcinoma (AC, 45%), squamous cell carcinoma (PE, 30%), and large cell carcinoma (LCC, 10%). The change in the frequencies of each histologic group was due in part to the introduction of filter cigarettes.
Most patients die from their disease, in part because 38% already have a metastatic stage at diagnosis [3]. In the early 1990s, the standard treatment for metastatic NSCLC was either “best supportive care” (BSC) or participation in a clinical trial. Fortunately, that has changed fundamentally. Thanks mainly to advances in molecular medicine, a whole range of new and very effective therapeutic options are now available.
Precise pathological diagnosis
Accurate histopathologic diagnosis is critical for the choice of therapy. The term NSCLC is not sufficient: on the one hand, a distinction must be made between AC, LCC and PE, and on the other hand, at least for all AC/LCC, molecular analyses are mandatory to search for a so-called driver mutation. Because the diagnosis is usually made during bronchoscopy or other small biopsy, it is challenging to obtain enough material for all of these tests. ASCO and other oncology societies have devised algorithms to ensure optimal use of valuable biopsy material [4].
It is important in this context that diagnostics can also be performed on cytological material. Recently, the use of “next generation sequencing” (NGS) has made comprehensive molecular analysis possible even with little material. NGS is certainly the methodology of the future [5]. In addition, new technologies make it possible to determine genetic changes in tumors from circulating free DNA in peripheral blood (“liquid biopsy”).
Therapy of squamous cell carcinoma
In metastatic PE, the first-line therapy of choice is a combination of a platinum with either gemcitabine or paclitaxel. This therapy allows a median survival of about 10-12 months, compared to 4-6 months without therapy or 8 months with a platinum and the older cytostatic agents [6]. Importantly, chemotherapy versus BSC also improves quality of life. If tolerance is acceptable and tumor progression is at least stable, 4(-6) cycles are appropriate [7]. Randomized trials have shown that neither prolonged therapy nor maintenance therapy lead to an improvement in overall survival (OS) [8]. Cisplatin has not shown a clear survival advantage over the less toxic carboplatin, but causes a higher response rate [9].
Therapy of adenocarcinomas without driver mutation
There are three important differences between ACs without mutations and PEs:
- A large randomized trial showed that combination therapy with a platinum and the folic acid analogue pemetrexed was the most effective therapy, both in terms of response rate and OS. The superiority of pemetrexed in AC over gemcitabine and the taxanes has also been demonstrated in other studies [6,10].
- In patients who demonstrate stable progression or response with four cycles of this therapy, maintenance therapy with pemetrexed alone may be considered. This can improve both progression-free (PFS) and OS, with OS exceeding one year with this therapy (13.9 months) [11]. Less common is “switch maintenance,” in which the patient is on a new substance during maintenance therapy. Effective agents are erlotinib and probably docetaxel [12,13].
- The additional use of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), may further improve survival, although only to a small extent [14].
Elderly patients and patients in reduced general condition
For a long time, it was unclear how to treat elderly patients and/or patients in reduced general condition (ECOG performance status 2-3). Two important randomized trials have helped here. An EORTC trial compared monotherapy with navelbine or gemcitabine with combination treatment with carboplatin and paclitaxel in patients over 70 years of age in good general health. Combination therapy produced a significant improvement in OS from 6.2 to 10.3 months [15]. Similarly, in a study comparing monotherapy with pemetrexed to a combination of carboplatin and pemetrexed in patients with poor general health (ECOG performance status of 2). Again, combination therapy was significantly better (median OS 5.3 vs. 9.3 months) [16]. Taking into account each patient’s individual situation, combination therapy should be sought whenever possible.
Adenocarcinomas with Driver mutation.
If a driver mutation is detected, the therapeutic strategy changes fundamentally. For these rare tumors, there are specific targeted tyrosine kinase inhibitors (TKIs) that provide a high response rate (60-80%) and long PFS with relatively good tolerability. An up-to-date listing of the most important mutations can be found at www.mycancergenome.org. The frequency of each mutation is shown in Figure 1.

EGFR mutation
Activating mutations of the epidermal growth factor receptor (EGFR) occur mainly in exon 19 and 21. For tumors with a deletion in exon 19, the EGFR TKI of choice is afatinib because it was the only TKI to show an OS benefit over first-line chemotherapy in a randomized trial, although a switch to a TKI was allowed if progression occurred in the chemotherapy arm [17]. Afatinib frequently causes diarrhea and skin toxicities. For mutations in exon 21 (most commonly the point mutation L858R), erlotinib or gefitinib are also established options. The rare mutations in exon 20 are usually associated with primary resistance to currently available TKIs.
Despite a median PFS of approximately one year on TKI, all patients develop resistance and disease progression. The gatekeeper mutation T790M is the cause of progression in almost 50% of all cases of resistance. The new TKI osimertinib is now available against this mutation with a response rate of at least 60% and a PFS of ten months, even after previous treatment with a standard TKI [18]. Some patients who become resistant to osimertinib have another gatekeeper mutation called C797S. However, no TKIs are yet available against this mutation [19].
Other resistance mechanisms include transformation to small cell carcinoma, which must then be treated as such, or the occurrence of MET amplification. Specific TKIs are also available for MET amplifications, including crizotinib, which is also effective against ALK-positive NSCLC. Rebiopsy after progression is strongly recommended because of the important therapeutic consequences!
Almost every patient with an EGFR mutation also receives conventional chemotherapy during the course of the disease; therapy of choice in this case is also a platinum and pemetrexed, as in AC without a driver mutation.
ALK translocation
4-7% of all AC have a translocation of the activated in lymphoma kinase (ALK) gene. A number of TKIs are available against these rare tumors. Approved crizotinib as first-line therapy: it results in a response rate of >70% and a PFS of 10.9 months [20]. Development of resistance is also inevitable with ALK mutations, but much more heterogeneous than with EGFR mutations. Approximately 50% of patients remain dependent on the ALK pathway (including resistance mutation of ALK), with some activation of alternative pathways occurring in the other patients.
In second-line therapy, ceritinib and alectinib proved effective with response rates around 60% and PFS of approximately seven months in phase II trials [21,22]. The most important side effect of ceritinib is nausea. Alectinib shows very good efficacy in the CNS, where metastases are most commonly located in ALK-positive AC. In secondary resistance, the major resistance mutation is the G1202R mutation, against which both ceritinib and alectinib are ineffective. Promising results have been observed in these tumors with lorlatinib, a third-generation ALK-TKI [23]. If chemotherapy is needed, pemetrexed and a platinum are also used for ALK-mutated NSCLC.
ROS1 translocation
The ROS1 translocation, a very rare genetic alteration, occurs in about 1.5% of all AC. Crizotinib is similarly effective as in ALK-positive AC and is therefore used as first-line therapy (however, it is not yet approved for this purpose) [24]. Ceritinib and alectinib have no effect against ROS1, whereas lorlatinib is effective.
MET overexpression
The oncogene “mesenchymal-epithelial transition factor” (MET) is the receptor for “hepatocyte growth factor” (HGF). MET overexpression or amplification is a relatively common secondary resistance mechanism with EGFR-TKI therapy (5-20%), but these changes also occur in untreated NSCLC (2-4%). For MET amplifications or the rarer exon 14 skip mutations, both crizotinib and cabozantinib are effective TKIs [25,26]. Newer TKIs, including INC280, are currently being studied in clinical trials.
Mutations with still unclear significance
Many NSCLC and especially AC have other rare mutations whose therapeutic significance is still unclear, such as BRAF and HER2 mutations and RET and NTRK1 translocations [27,28]. Specific TKIs exist for all of these genetic aberrations, and it is hoped that highly effective therapeutic strategies can be found in the near future.
A major exception is the KRAS mutation, by far the most common mutation in AC at 20-25%. Unfortunately, there are no therapeutic approaches for these at present. Patients with KRAS-mutated NSCLC are treated with conventional chemotherapy but have a worse overall prognosis than other patients.
Immunotherapies
The most important innovation in the last two years is the introduction of immunotherapy. The second generation of immunotherapies consists of antibodies directed against either PD-1 or its ligand PD-L1, leading to activation of cytotoxic T cells in the tumor. Randomized trials in second-line NSCLC have shown superiority of these so-called immune checkpoint inhibitors over standard chemotherapy with docetaxel for both PE and AC [29,30]. They not only achieved a better response rate, but also a longer PFS and OS. There is even hope that a long and possibly sustained remission can be achieved in some patients. Thus, in PE treated with nivolumab, median PFS was 3.5 months and 1-year OS was 42% compared with 2.8 months and 21% with docetaxel.
Immunotherapies appear to work better for tobacco-induced carcinomas than for tumors with a driver mutation. Induction of so-called neo-antigens by toxic tobacco exposure is thought to play a role. These neo-antigens offer themselves to the immune system as new targets. The best studied drugs to date are nivolumab (approved in Switzerland as second-line therapy) and pembrolizumab.
Conclusion
New findings in recent years have significantly improved the prognosis of NSCLC. Advances in both TKIs and immunotherapies have been rapid. A differentiated therapy alogrithm can be derived from the new findings (Fig. 2).
Literature:
- Lozano R, et al: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859): 2095-2128.
- Arndt V, et al: Swiss Cancer Report 2015: Status and Developments. Neuchatel: Swiss Federal Statistical Office; 2015.
- Morgensztern D, et al: Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010; 5(1): 29-33.
- Leighl NB, et al: Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors. J Clin Oncol 2014; 32(32): 3673-3679.
- Vigneswaran J, et al: Comprehensive genetic testing identifies targetable genomic alterations in most patients with non-small cell lung cancer, specifically adenocarcinoma, single institute investigation. Oncotarget 2016, Feb 26. doi: 10.18632/oncotarget.7739. [Epub ahead of print]
- Scagliotti GV, et al: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in che-motherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26(21): 3543-3551.
- Rossi A, et al: Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2014; 15(11): 1254-1262.
- Brodowicz T, et al: Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 2006; 52(2): 155-163.
- de Castria TB, et al: Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2013; 8: CD009256.
- Scagliotti G, et al: Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011; 6(1): 64-70.
- Paz-Ares LG, et al: PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31(23): 2895-2902.
- Cappuzzo F, et al: Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11(6): 521-529.
- Fidias PM, et al: Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009; 27(4): 591-598.
- Soria JC, et al: Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013; 24(1): 20-30.
- Quoix E, et al: Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011; 378(9796): 1079-1088.
- Zukin M, et al: Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013; 31(23): 2849-2853.
- Yang JC, et al: Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16(2): 141-151.
- Janne PA, et al: AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372(18): 1689-1699.
- Niederst MJ, et al: The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 2015; 21(17): 3924-3933.
- Solomon BJ, et al: First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371(23): 2167-2177.
- Shaw AT, et al: Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370(13): 1189-1197.
- Gadgeel SM, et al: Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014; 15(10): 1119-1128.
- Shaw A, et al: Clinical activity and safety of PF-06463922 from a dose escalation study in patients with advanced ALK+ or ROS1+ NSCLC. J Clin Oncol 2015; 33(suppl; abstr 8018).
- Shaw AT, et al: Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014; 371(21): 1963-1971.
- Ross Camidge D, et al: Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol 2014; 32(5s, suppl; abstr 8001).
- Paik PK, et al: Response to crizotinib and cabozantinib in stage IV lung adenocarcinoma patients with mutations that cause MET exon 14 skipping. J Clin Oncol 2015; 33(suppl; abstr 8021).
- Mazieres J, et al: Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016; 27(2): 281-186.
- Peters S, et al: Dramatic response induced by vemurafenib in a BRAF V600E-mutated lung adenocarcinoma. J Clin Oncol 2013; 31(20): e341-344.
- Brahmer J, et al: Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373(2): 123-135.
- Borghaei H, et al: Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373(17): 1627-1639.
- Boolell V, et al: The Evolution of Therapies in Non-Small Cell Lung Cancer. Cancers (Basel) 2015; 7(3): 1815-1846.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(3): 6-10.