Hidradenitis suppurativa (HS) has a multifactorial etiopathogenesis and is associated with high impairment of quality of life. Empirical findings of increased expression of inflammatory cytokines suggest an immunologic genesis of this inflammatory recurrent skin disease. The range of drug treatments available to date is limited. In parallel with further research into the immunopathological basis, various active substances are being tested in clinical trials.
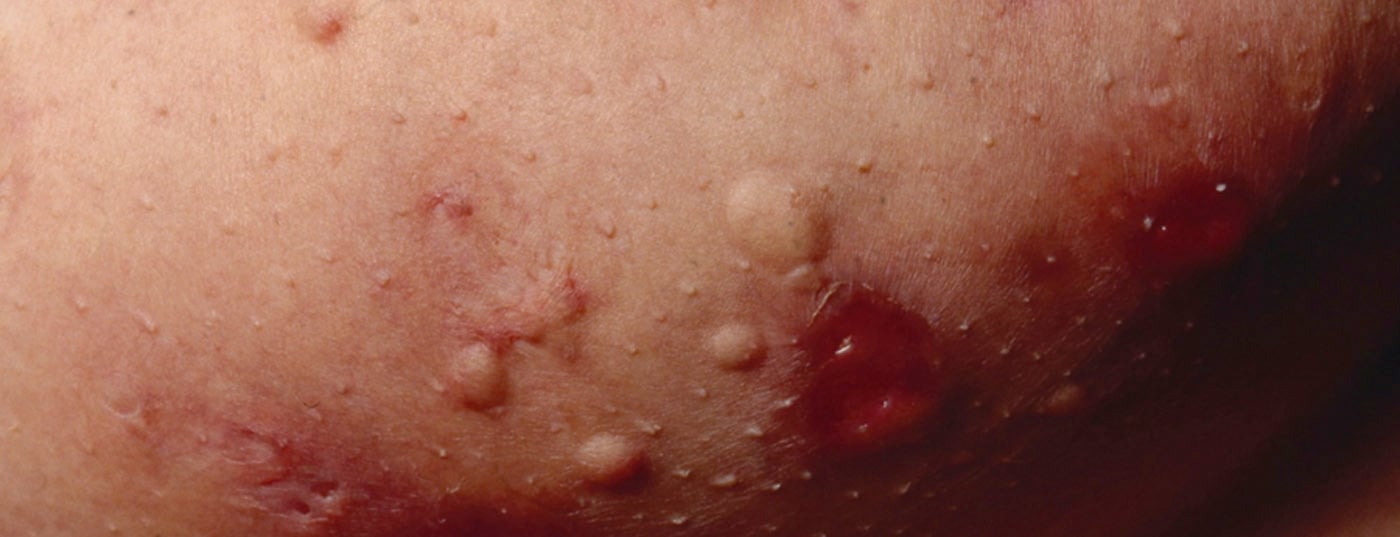
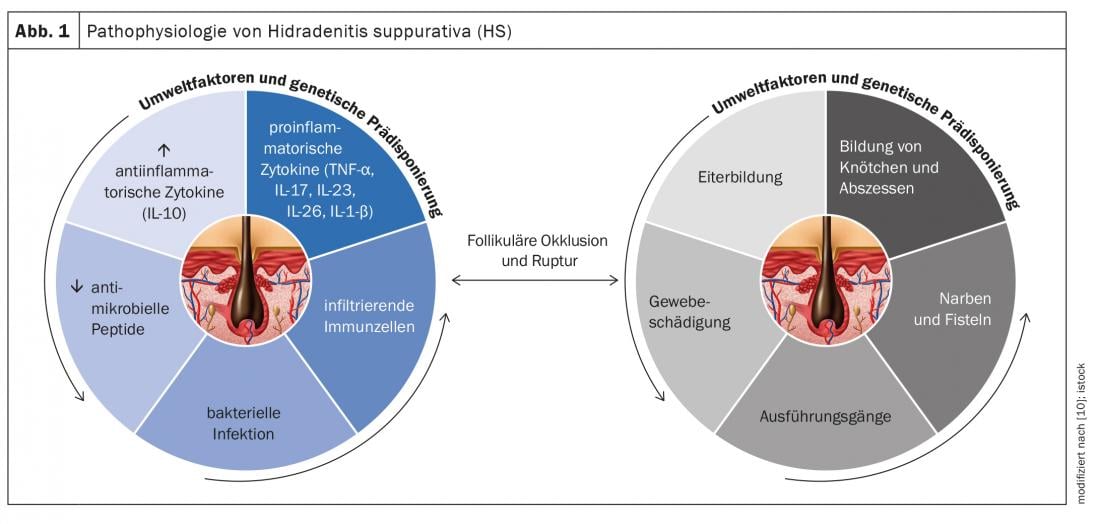
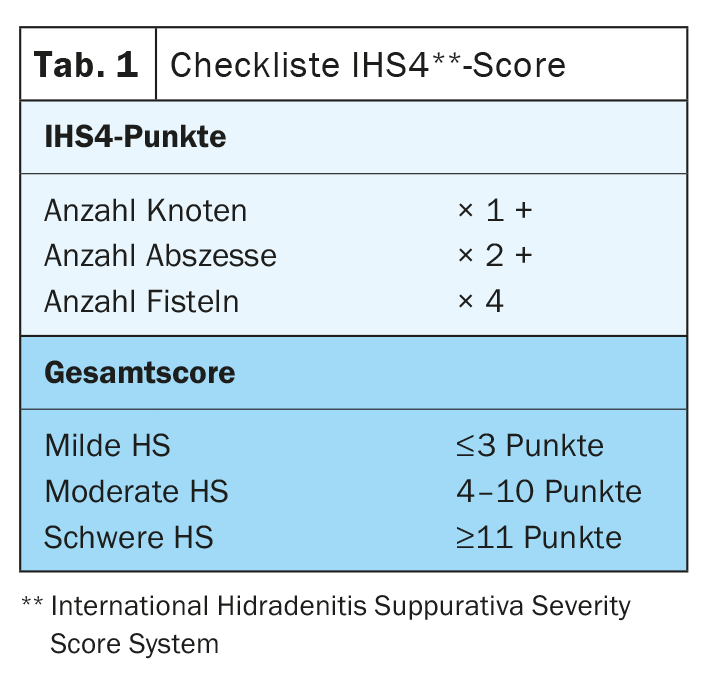
Hidradenitis suppurativa (HS) – also called “acne inversa” – is a chronic inflammatory disease of the hair follicles that leads to chronic fistulas, abscesses, and scarring in the axillary, inguinal, and perianal regions [1]. A multifactorial pathogenesis is thought to exist, although many unanswered questions remain [2]. According to current knowledge, HS begins with occlusion and dilation in the hair follicle, leading to rupture and an inflammatory response, resulting in chronic inflammation with fistula tracts [3,4]. Numerous cytokines are involved in the multifactorial inflammatory response. In addition to genetic predisposing factors, smoking and obesity contribute to disease development (Fig. 1) [11]. Depending on the clinical manifestation, different degrees of severity are distinguished. In addition to Hurley’s classification [5], Zouboulis et al. In 2017, the International Hidradenitis Suppurativa Severity Score System (IHS4 score) was published – a validated tool for assessing the severity of HS that can be used in clinical practice(Table 1) [6,9].
Need for additional drug therapy options
Treatment options for HS are limited. There are several approaches to control inflammation and reduce the severity of manifestations. Antiseptic and antibiotic creams and solutions, as well as antibiotics in tablet form, can reduce germs on the skin, helping to improve patients’ quality of life. The biologic adalimumab (Humira®), a tumor necrosis factor (TNF)-α blocker, has been approved in Switzerland since 2016 for the treatment of intermediate and advanced stage HS [7]. In addition, various pharmacological therapies are used off-label. Other treatment options include laser medical procedures and surgical excision of the fistulas with or without plastic defect coverage.
Pathophysiology of HS: immunopathological findings at a glance.
In order to expand the spectrum of drug treatment, research into the pathophysiological basis of HS has increasingly come into scientific focus in recent years. Here, the role of various proinflammatory cytokines is analyzed at the molecular level [2]. The following are the results of a secondary analysis published in the International Journal of Molecular Sciences in 2020 with an integration of immunopathological findings in HS patients [2]:
TNF-α and IFN-γ: Up-regulated TNF-α levels show a positive correlation with the severity of HS. TNF-α increases the ratio of Th17:Treg*, resulting in excessive production of Th17 cells and, accordingly, the cytokines IL-17 and IL-22. TNF-α induces the expression of chemokines CXCL8, CXCL11, CCL20, and CCL2 in keratinocytes. These are responsible for the recruitment of neutrophils, T cells, and monocytes to the skin. Overall, these signals lead to massive infiltration of immune cells into damaged tissue. Therefore, HS lesions are characterized by granulocytes, T cells, B cells, and monocytes, which differentiate into macrophages and dendritic cells. IL-23 and IL-12 support Th17 and Th1 cells in the production of the cytokines IL17 and IFN-γ.
*Treg=regulatory T cells
IL-1: Empirical findings show that IL-1-mediated processes are upregulated in HS and contribute to cell infiltration and tissue damage. The IL-1 cytokine family includes 7 cytokines with pro-inflammatory activity, which include IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ. IL-1α is strongly pro-inflammatory and induces a cascade of inflammatory mediators such as TNF and IL-18. Several empirical findings indicate that keratinocytes produce elevated levels of IL-1. A positive feedback mechanism is thought to exist between IL-1 and IL-17. IL-36 is involved in the activation of inflammasomes as well as proinflammatory signaling by activating nuclear factor-kB (NF-κB) and mitogen-activated protein kinase (MAPK). Increased levels of IL-36α, IL-36β, and IL-36γ were detected in the serum and lesional skin of HS patients.
IL-6: The evidence on the associations between IL-6 and HS is controversial. While some studies demonstrated increased IL-6 mRNA expression in lesions of HS patients compared with non-lesional skin, other studies showed decreased IL-6 levels in HS lesions compared with non-lesional skin. IL-6 is a pleotropic cytokine that plays a key role in a wide range of immune processes. In combination with TGF-β, IL-1β, and IL-23, IL-6 triggers the development of Th17 cells and inhibits the development of TGF-β-induced regulatory T cells.
IL-10: It has been observed that in HS not only are there elevated levels of proinflammatory cytokines, but also the anti-inflammatory mediator IL-10 is overexpressed in lesional skin of HS patients. One possible explanation is that the upregulation of the immunosuppressive IL-10 is a compensatory response to inflammatory processes and the spread of commensal microbes in the skin. This suppresses IL-22 and IL-17 levels in lesional skin. To find out more about the role of this anti-inflammatory cytokine in the pathogenesis of HS, further studies are needed.
IL-17/IL-23: In the papillary and reticular dermis of HS lesions, there are a large number of Th17 cells, which may be involved in excessive neutrophilic inflammation and purulent drainage. Overexpression of IL-17 was observed in lesional, perilesional, and nonlesional skin of HS patients, indicating subclinical inflammation preceding the formation of active lesions. Increased levels of IL-17 have also been detected in the serum of HS patients. In keratinocytes, IL-17 induces the expression of several proteins (LL37/cathelicidin, S100A7, S100A8, and S100A9), which are increased in lesional skin and serum of HS patients but not in perilesional skin. These proteins are involved in keratinocyte proliferation and expression of proinflammatory cytokines and chemokines. Overall, the findings suggest that the IL-23/IL-17 axis is significantly involved in the pathogenesis of HS.

In parallel with research into pathomechanisms at the molecular level, clinical trials are investigating the efficacy of new anti-inflammatory therapies [8]. This includes a case series presented at this year’s DDG meeting by Caroline Hilbring, MD, assistant physician, Institute for Health Services Research in Dermatology and the Nursing Professions, University Hospital Hamburg (D) (box ) [9].
Congress: DDG Conference 2021
Literature:
- Iesalnieks I, Dornseifer U: Acne inversa (hidradenitis suppurativa). Surgeon 2020; 91: 293-300.
- Del Duca E, et al. : Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int J Mol Sci 2020; 21: 8436; doi:10.3390/ijms21228436.
- Vossen ARJV, van der Zee HH, Prens EP: Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol 2018; 9: 2965.
- Van der Zee H: Biologics in the treatment of hidradenitis suppurativa. Hessel van der Zee, MD, PhD. EADVVirtual Highlights, 2020.
- https://2020.eadvhighlights.com (last accessed 14.09.2021)
- Revuz J: Hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2009; 23: 985-998.
- Zouboulis C, et al: Development and validation of the International Hidradenitis Sup-purativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017; 177: 1401-1409.
- Drug Information, www.swissmedicinfo.ch, (last accessed Sept. 14, 2021).
- Schuch A, Absmaier-Kijak M, Volz T: Acne inversa/Hidradenitis suppurativa – From pathogenesis to therapy. Current Dermatology 2019; 45(06): 277-287.
- Hilbring C, Kirsten N, Augustin M: Case series of 10 patients with acne inversa: use of Ixe-kizumab in secondary loss of effect of adalimumab. FV01/08, DDG Meeting, 04/15/2021.
- Scala E, et al: Hidradenitis Suppurativa: Where We Are and Where We Are Going. Cells 2021; 10(8), 2094; https://doi.org/10.3390/cells10082094
- Wolk K, Join-Lambert, O, Sabat, R: Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol 2020; 183(6): 999-1010.
DERMATOLOGIE PRAXIS 2021; 31(6): 39-40