Stereotactic surgery in the treatment of idiopathic Parkinson’s disease is a promising therapeutic option. Research status, use and basic principles of deep brain stimulation (DBS) and MR-guided focused ultrasound (MRgFUS) methods.
Last year marked the 200th anniversary of the first description of Parkinson’s disease by James Parkinson in 1817. It is impressive how well Parkinson’s syndrome can still be diagnosed today based on the six cases he described with their respective motor and non-motor symptoms. However, although great progress has been made in recent decades in the study of the pathophysiology of idiopathic Parkinson’s disease, the cause of the disease is still unknown and, accordingly, the disease cannot be cured.
Categorization and clinic
In movement disorders, we distinguish hyperkinetic (tremor, dystonia, myoclonus, chorea, and tic) and hypokinetic movement disorders, the main representative of the latter category being Parkinson’s syndrome. Motor Parkinson’s disease requires the presence of rigor, tremor (typically a resting tremor that increases with cognitive activity and disappears when movement is initiated), and generalized slowing in the sense of bradykinesia. In recent decades, however, we have become aware that both patients and relatives additionally suffer from non-motor symptoms. These include disorders of the sense of smell, REM sleep behavior disorders, autonomic disorders (sexual dysfunction, bladder dysfunction, blood pressure fluctuations, gastrointestinal complaints such as constipation and delayed gastric emptying), and psychiatric complaints (depression, hallucinations, fatigue, etc.) [1].
1% of all people over 60 years of age suffer from idiopathic Parkinson’s syndrome; in Switzerland, more than 15,000 people are affected by this disease [2]. However, it is by no means only older people who develop the disease, even though old age remains the greatest risk factor for idiopathic Parkinson’s syndrome. In our movement disorder consultations, our experience is that approximately 10% of patients develop the disease before the age of forty.
Diagnosis
For reasons that have not yet been explained, there is a loss of dopaminergic neurons in particular, which can also be objectified by nuclear medicine using DaTSCAN-SPECT in terms of a presynaptic dopaminergic deficit. As a rule, however, the diagnosis must be made purely on the basis of clinical neurology and anamnesis and after excluding other causes. By the time motor Parkinson’s syndrome manifests, more than 50% of the dopaminergic cells have already perished.
Important differential diagnoses of idiopathic Parkinson’s syndrome are atypical Parkinsonian syndromes such as multisystem atrophy (here, there is additional evidence of involvement of other neurological systems, e.g., in the form of pyramidal tract syndrome, cerebellar syndrome, and early autonomic dysfunction) and progressive supranuclear gaze palsy (PSP) with its various subtypes. Corticobasal degeneration should also be considered as a differential diagnosis. If idiopathic Parkinson’s disease and multisystem atrophy are alpha-synucleinopathies, PSP or corticobasal degeneration neuropathologically underlies tauopathy. Important differential diagnosis is also drug-induced parkinsonoid due to neuroleptic therapy or normal pressure hydrocephalus. So far, Parkinson’s syndromes resulting from manganese poisoning, as described mainly in polytoxicomania, have not occurred in Switzerland. In younger patients, Wilson’s disease should be excluded as a treatable condition.
Drug therapy
The gold standard of therapy continues to be drug therapy, especially by administration of L-dopa in its pharmacological variants and always in combination with a decarboxylase inhibitor to prevent premature degradation in the periphery. Dopamine agonists such as pramipexole, rotigotine, or ropinirole are also used with success, but are not as effective as L-dopa itself. COMT inhibitors (entacapone, tolcapone) and MAO-B inhibitors (rasagiline or safinamide) are also used. Rarely used are anticholinergics, which tend to cause delirium on the floor of neurodegenerative disease. In crisis situations, intravenous amantadine may be administered. As a rough rule, patients before the age of seventy are primarily started on dopamine agonist therapy, and those with disease over the age of seventy are primarily started on L-dopa. In principle, however, the patient’s quality of life should be paramount. If sufficient clinical improvement cannot be achieved with the administration of a dopamine agonist, we generously switch to the most effective therapy in the form of L-dopa. Initially, important side effects include nausea, circulatory disturbances, leg edema (mainly due to dopamine agonists), fatigue, and psychiatric side effects such as hallucinations. The danger of impulse control disorders should be emphasized. Sometimes gambling addiction, hypersexuality, increased shopping, and compulsive repetitive activities may occur. This serious side effect can break up families and it is therefore the duty of every doctor who prescribes these drugs to also ask about this form of side effect. At the beginning, patients with idiopathic Parkinson’s syndrome respond very well to therapy, so that one can speak of a phase of “honeymoon”, which, however, can turn into a “lost paradise” over time. The initial good effect changes. There are fluctuations in effect over the course of the day, a delayed or absent “on,” L-dopa-induced dyskinesias, dystonia as L-dopa deficiency syndrome (typically in the morning), or psychiatric side effects. At the latest in this situation, the possibility of intervening therapies must be considered. On the one hand, the possibility of an apomorphine pump should be mentioned. Here, apomorphine is continuously applied subcutaneously via a pump system. Apomorphine is the most potent dopamine agonist, but it can only be administered parenterally. Intrajejunal continuous infusion of L-dopa is another alternative. Due to continuous dopaminergic stimulation, there is a decrease in effect fluctuations and improvement in L-dopa-induced dyskinesias with both therapies [2].
Deep Brain Stimulation (DBS)
Other possible invasive therapies available to us include stereotactic surgery such as deep brain stimulation (DBS) and, more recently, MR-guided focused ultrasound (MFgFUS). In the eighties of the last century, neurologists and neurosurgeons from France, but also from Switzerland, tried to establish this new method. It is fair to say that this is the biggest therapeutic step in the treatment of Parkinson’s disease since the introduction of L-dopa in the 1960s.
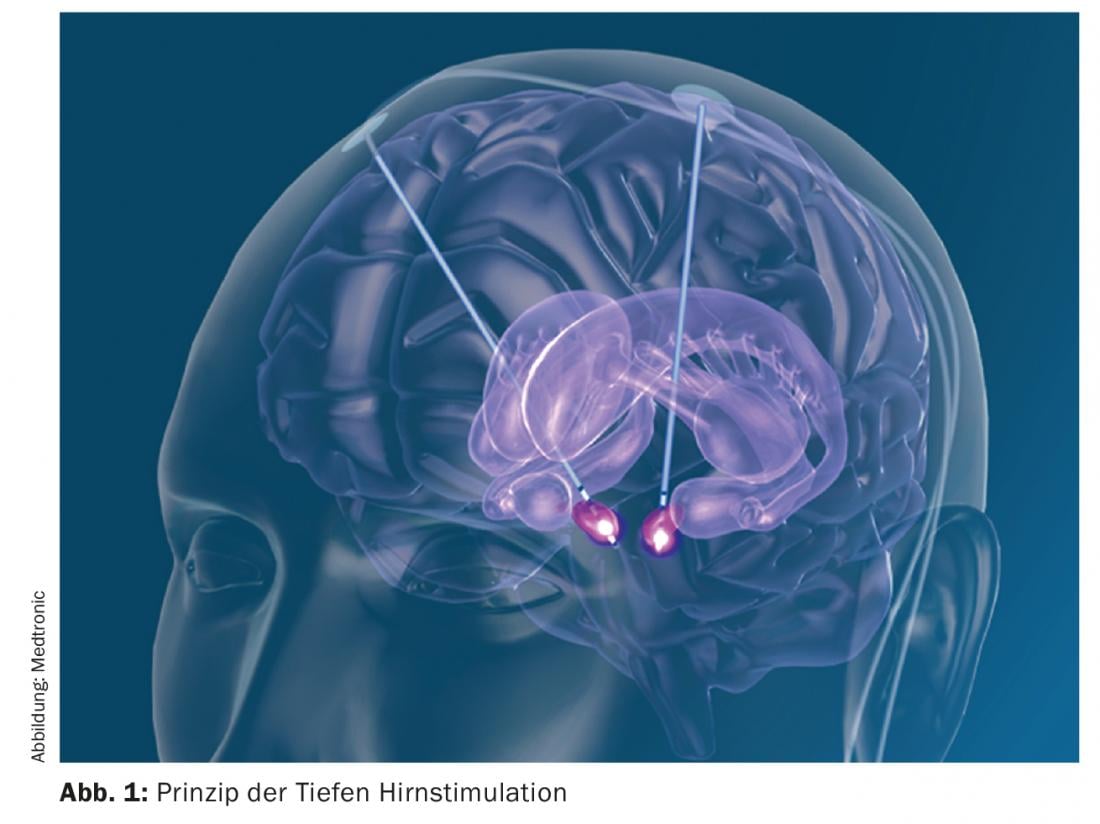
Deep brain stimulation is a non-lesional method in which electrodes are implanted at a specific target point during stereotactic surgery. Subsequently, cables are laid under the skin and a neurostimulator is implanted subclavicularly or abdominal. The parameters that enable neurostimulation to be performed can be set externally (Fig. 1) . It should be emphasized that neurostimulation does not destroy tissue, but only functionally inactivates it. Typical targets in the treatment of Parkinson’s disease are the subthalamic nucleus (STN), the globus pallidus internus (Gpi), or specific nuclear areas of the thalamus. Both the STN and Gpi produce improvement in all the cardinal symptoms of Parkinson’s (rigor, bradykinesia, and tremor). In the case of neurostimulation of the STN, it is possible to reduce dopaminergic therapy by approximately half and thus improve L-dopa-induced dyskinesias. Neurostimulation in the nucleus ventralis intermedius Thalami (Vim) results in a reduction of tremor without affecting the other cardinal symptoms. During stereotactic surgery, the patient, who is usually awake, is fixed in a stereotactic frame system to allow implantation of the electrodes with millimeter precision. With careful preparation and patient selection, it is a surgical procedure with comparatively few side effects [3]. Possible surgery-related side effects include intracranial hemorrhage, infection and skin erosions, the triggering of epileptic seizures, the development of a cerebrospinal fluid fistula or, in the worst case, direct damage to brain tissue. However, because the surgery can be specifically planned with software, these side effects are less than 3%, depending on the study. In addition, there are possible dysfunctions of the neurostimulator system such as migration of the electrodes, rupture or malfunction, but this is also extremely rare due to the very reliable systems. In the depth of the brain, important neuronal systems run very close to each other, including at the aforementioned target points of deep brain stimulation. Accordingly, stimulation-induced side effects may also occur. It is the art of the treating neurologist to find the right setting in the multitude of possible settings so that the desired effect occurs and stimulation-related side effects such as speech disorders, cramps of the contralateral hand, insensations, etc. are avoided, which is usually done well. Like drug therapy, neurostimulation can cause psychiatric side effects. These are also very rare and reversible. As with any brain surgery, especially in people suffering from neurodegenerative disease, postoperative delirious syndromes are temporarily possible. However, direct stimulation can also lead to a certain increase in drive and hypomanic behavior as well as hypersexuality due to the co-irritation of limbic fibers. Also, in order to recognize these side effects, it is important to involve relatives in the treatment process from the beginning.
Recently, directional electrode systems have also become available. These make it possible to stimulate in specific directions and thus avoid or correct stimulation-induced side effects.
Careful patient selection as a prerequisite
The method stands and falls with careful patient selection, for which those involved must take sufficient time. Patients with idiopathic Parkinson’s syndrome are particularly suitable. The atypical parkinsonian syndromes previously mentioned as differential diagnoses do not respond comparably to DBS. Only patients who benefit from treatment with L-dopa will also experience improvement with deep brain stimulation. This makes it possible to predict the therapeutic effect. Only the cardinal symptom, tremor, does not necessarily improve with treatment with L-dopa, but is an excellent target symptom for DBS. Today, we can speak of DBS as an established therapy for idiopathic Parkinson’s disease, which leads to an improvement in daily activity through regulation of motor functions, via a reduction in daily dopaminergic therapy, to a reduction in L-dopa-induced dyskinesias, and thus to an overall improvement in quality of life. Controlled studies are available showing an advantage of DBS over a comparable patient population receiving “only” drug treatment [3]. Accordingly, the possibilities of DBS should be considered early in the course of the disease, as this therapy is already indicated when the corresponding indication is present (effect fluctuations, dyskinesias) [4].
MR-guided focused ultrasound (MRgFUS).
In addition to DBS as a non-lesional stereotactic therapeutic procedure, lesional stereotactic procedures also exist. However, these have become less important than DBS in recent decades due to their irreversibility. Radiofrequency ablation, radiosurgery with gamma knife, and, most recently, MR-guided focused ultrasound (MRgFUS) are available [5].
Ultrasound technology, which is otherwise used for diagnostic purposes, can be used for millimeter-precise lesions without having to open the skullcap thanks to a technical processing of the sound energy. The system contains over 1000 ultrasound sources and can be used integrated in a 3T MRI (Fig. 2). Only the Exablate Neuro system from the Israeli company InSightec is approved for clinical use. Prior to the procedure, the patient’s head is completely shaved to ensure adequate transmission of ultrasound energy. In the MR center, analogous to DBS, the stereotactic frame is placed under local anesthesia and the patient is fixed on the treatment couch by means of the stereotactic frame. Mounted on this is the Exablate neuro-treatment system, which is integrated with a 3T MRI system. The entire procedure is performed on the awake patient and is accompanied by an anesthesiology team. According to our experience, pain therapy in awake patients during DBS surgery is performed only with remifentanil, if possible, in order not to influence the target symptoms.

The space between the hemispherical transducer and the patient’s scalp is filled with degassed water at 16°C, which is circulated to transmit sound and cool the scalp locally; a silicone membrane is used to seal it to the patient’s scalp. In the following, current MRI sequences are fused with previously prepared planning sequences. The target point is determined neurosurgically and neuroradiologically with the inclusion of stereotactic atlases and direct targeting based on the patient’s individual anatomy. With the beginning of the actual intervention, sonications (sonications) with low sonication energy are initially performed for ten seconds at a time. Between sonications, clinical testing of the target symptom and possible side effects is performed by the neurologist. During each sonication, the temperature development in the target voxel and its localization according to plan is monitored on the control console using corresponding MRI sequences. Together with the clinical feedback from the neurologist, before gradually increasing the applied sound energy and duration (max. 30,000 J over max. 30 seconds) and thus the increase of temperature a correction of sound energy as well as target point position is possible. In this way, the target temperature of 56-60°C, which causes irreversible coagulation of the tissue, is reached in a gradual and controlled manner. This procedure allows the safe testing of the clinical effect and possible side effects as well as the adjustment of the target point before the final, irreversible ablation is performed.
Immediately upon reaching a temperature threshold between 48-55°C, an anatomical as well as clinical effect is evident, on the basis of which the further procedure can be planned. Depending on the clinical response to the target symptom, image morphologic evidence of the extent of a lesion in the target site, and documentation of the target temperature achieved, an interdisciplinary decision is made as to when to terminate therapy [6].
An advantage of MRgFUS is that during the intervention in MRI the target point can be planned and directly also checked for side effects or the therapy effect can be monitored. Opening of the skullcap is not necessary and risks of infection are therefore meaningless. The lesion placed with MRgFUS reaches a size maximum after approximately 72 hours and then, in our experience, regresses in six months to one year such that the lesion is almost undetectable on MRI. This is an advantage over other lesions, in some of which an increase in size during the course has been described.
Several anatomic targets are defined in the posterior subthalamic region, which are candidates for stereotactic treatment, either with DBS or a lesional procedure. Which target to choose depends on the underlying disease. In our experience, not only idiopathic Parkinson’s disease but also essential or dystonic tremor may be an indication for treatment with MRgFUS. In 2016, a randomized and sham-controlled trial was conducted, which showed a significant advantage of the treated patient group over the sham-treated patients in terms of tremor reduction [7]. In general, tremor seems to be a particularly good target symptom for treatment with MRgFUS. The tremor-reducing effect also proved stable in the follow-up study after two years. We ourselves had good experience with patients suffering from essential tremor syndrome. We used the cerebello-thalamic tract (fasciculus cerebello thalamicus, FCT) as a target, which resulted in over 80% improvement in symptoms [8]. So far, the intervention is performed only unilaterally in our center to avoid possible bilateral side effects. Postintervention, some patients showed transient mild gait unsteadiness as side effects and headache and nonspecific dizziness during the intervention. However, we did not record any long-term side effects, especially dysarthrophonia or dysphagia.
In the much more severely affected and often more morbid patients suffering from advanced idiopathic PD, careful patient selection is essential. Sometimes there is confusion about the target structure to be chosen for a lesion with MRgFUS. If the goal is pure tremor reduction, the FCT as a cerebellar afferent to the thalamus can also be lesioned unilaterally in these patients, resulting in contralateral tremor reduction. However, the other cardinal symptoms such as rigor and bradykinesia are not affected by this and it is unclear to what extent postintervention there is a possibility to reduce the medication in order to avoid L-dopa-induced dyskinesias as well.
At our center, we have also been able to gain experience with the treatment of Parkinson’s patients. Most of these were patients who were not eligible for DBS with electrodes for various reasons. In these patients, we chose the pallido-thalamic tract (PTT) as the target site. These are fibers from the pallidum that converge onto the thalamus, making them readily accessible to lesional treatment. Here, too, our experience was that tremor-dominant syndromes in particular experienced a marked improvement that also lasted for several years. In addition, some patients experienced an improvement in bradykinesia as well as rigor, so that an improvement in other cardinal symptoms can also be hoped for. However, further experience in the context of clinical trials and long-term observations in the context of a study ongoing at our center are needed to assess this over time [8].
Recently, a group from Madrid used MRgFUS in patients with severely asymmetric idiopathic Parkinson’s disease, targeting the subthalamic nucleus as the lesion site. The goal was therefore the same target point as in the DBS described above. According to the data available to date, there was also improvement in contralateral parkinsonism without lesion-related hyperkinesia [9].
Overall, it can be stated that treatment with MRgFUS is a new and very promising therapeutic option for movement disorders in the future. It is a lesional and therefore irreversible procedure. The intervention itself, however, is noninvasive, and some surgery-related side effects that we are familiar with in DBS do not play a role in this procedure. In progressive neurodegenerative diseases such as idiopathic Parkinson’s syndrome, it is not possible during the course to adapt the effect achieved by the therapy to the progression of the disease, since a new lesion would be required here.
However, compared to the large body of experience on DBS available through controlled trials, further experience with this therapy has yet to be gained.
Conclusion
Stereotactic surgery in the treatment of idiopathic Parkinson’s disease as well as other movement disorders is an invasive but promising therapeutic option. This offer should be made available to our patients in advanced stages and after all drug therapy options have been exhausted. With careful patient selection and pretesting, the procedures have comparatively few side effects.
Take-Home Messages
- Although drug therapy remains the gold standard, stereotactic surgery is a promising therapeutic option in the treatment of idiopathic Parkinson’s disease.
- Careful patient selection is essential for therapeutic success.
- Deep brain stimulation (DBS) is a non-lesional method in which neurostimulation is performed via implanted electrodes.
- MR-guided focused ultrasound (MRgFUS) is a lesional, irreversible procedure. Because the intervention does not require opening of the skull or implantation of foreign material, some surgery-related side effects are eliminated.
- MRgFUS appears to be most successful in tremor-dominant syndromes.
Literature:
- Postuma RB, Berg D: The New Diagnostic Criteria for Parkinson’s Disease. Int Rev Neurobiol 2017; 132: 55-78.
- Diener HC, et al: Guidelines for diagnosis and therapy in neurology: published by the Guidelines Commission of the German Neurological Society, 5th edition. Stuttgart 2012.
- Deuschl G, et al: A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006; 355: 896-908.
- Schuepbach WM, et al: Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med 2013; 368(7): 610-622.
- Schreglmann SR, et al: Functional lesional neurosurgery for tremor: back to the future? J Neurol Neurosurg Psychiatry 2017; 210: 1-9.
- Schreglmann SR, et al: Focused ultrasound ablation as tremor treatment. Nervenarzt 2018 [Epub ahead of print].
- Elias WJ, et al: A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med 2016; 375(8): 730-739.
- Schreglmann SR, et al: Unilateral cerebellothalamic tract ablation in essential tremor by MRI-guided focused ultrasound. Neurology 2017; 88: 1329-1333.
- Martínez-Fernández R, et al: Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol 2018; 17(1): 54-63.
InFo NEUROLOGY & PSYCHIATRY 2018; 16(4): 6-10.











