Fungal infections are mostly caused by dermatophytes, but also by fungi and more rarely by molds. The basis for effective treatment is the species-specific and timely identification of the pathogen by means of mycological diagnostics. The topical and oral antifungal therapies available today are effective in most patients when used regularly and as prescribed.
Human pathogenic dermatophytes are keratinolytic filamentous fungi of the genera Trichophyton, Microsporum, Nannizzia, and Epidermophyton [1]. Among the pathogens are anthropophilic species that have adapted to the human immune system and are characterized by a chronic course with limited spread and minimal inflammatory response; important representatives are. Trichophyton (T.) rubrum and T. interdigitale. Furthermore, zoophilic dermatophytes (especially Microsporum [M .] canis and increasingly Arthroderma [A .] benhamiae) of importance, which are transmitted from animals and often cause highly inflammatory lesions. However, rarer dermatophytes such as Epidermophyton (E.) floccosum, T. verrucosum , and geophilic Nannizzia species and “emerging pathogens” such as T. erinacei may also be present in everyday clinical practice.
The following is an overview of the clinical picture and the diagnosis and therapy of skin and nail mycoses, with an emphasis on infections caused by dermatophytes.
Dermatophytoses – clinical picture
Dermatophytosis usually presents as eczema-like changes with redness, scaling, and itching that are sometimes clinically indistinguishable from eczema of other causes. Typically, disc-shaped foci occur with sharp margins, a slightly elevated, scaling rim, and healing in the center. In the case of scaly skin lesions, mycological diagnostics should always be performed to avoid overlooking a mycosis.
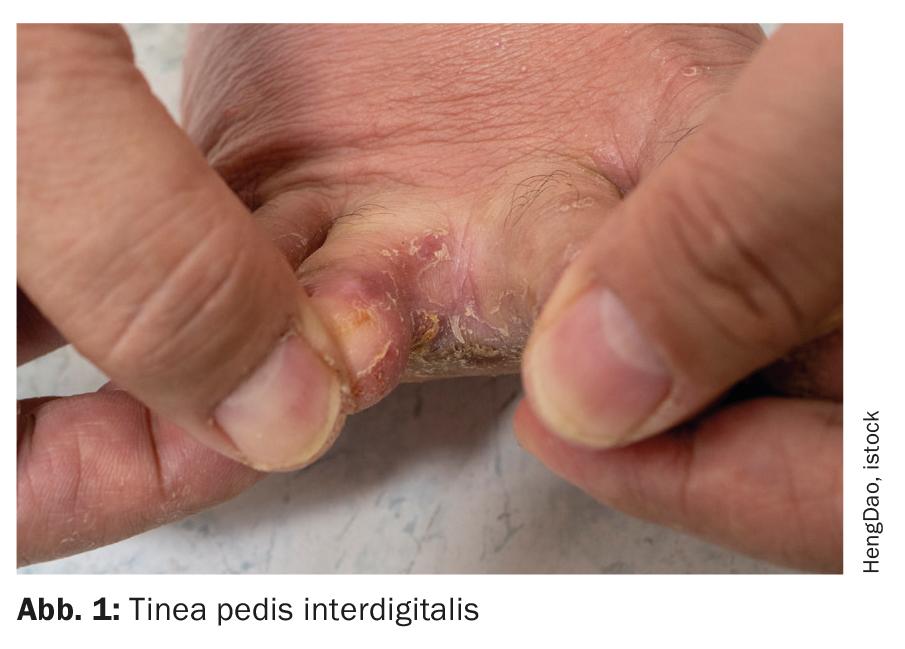
Clinically, dermatophytoses are classified according to the areas of the body affected. In industrialized countries, tinea pedis (“athlete’s foot”, Fig. 1) the most common disease caused by fungal infection, followed by tinea corporis and infestation of the nails (tinea unguium). In children, tinea capitis is significant. Common causative agents of tinea capitis are Trichophyton tonsurans (Fig. 2).
Tinea pedis initially appears mostly between the toes in the form of hyperkeratotic, dry and scaly lesions that later become macerated, weeping and erosive. The infection often spreads to the sole and arch of the foot, typically being sharply demarcated at the edge of the foot (“moccasin mycosis”). Interdigital mycosis can also spread to the dorsum of the foot as well as the toenails. It is not uncommon for tinea pedis to lead to self-infection of other areas of the body. Pathogens are mainly anthropophilic dermatophytes such as T. interdigitale, T. rubrum and E. floccosum. It is estimated that about one third of Europeans are affected by this mycosis. Infection often occurs in swimming pools and saunas or through contaminated footwear.
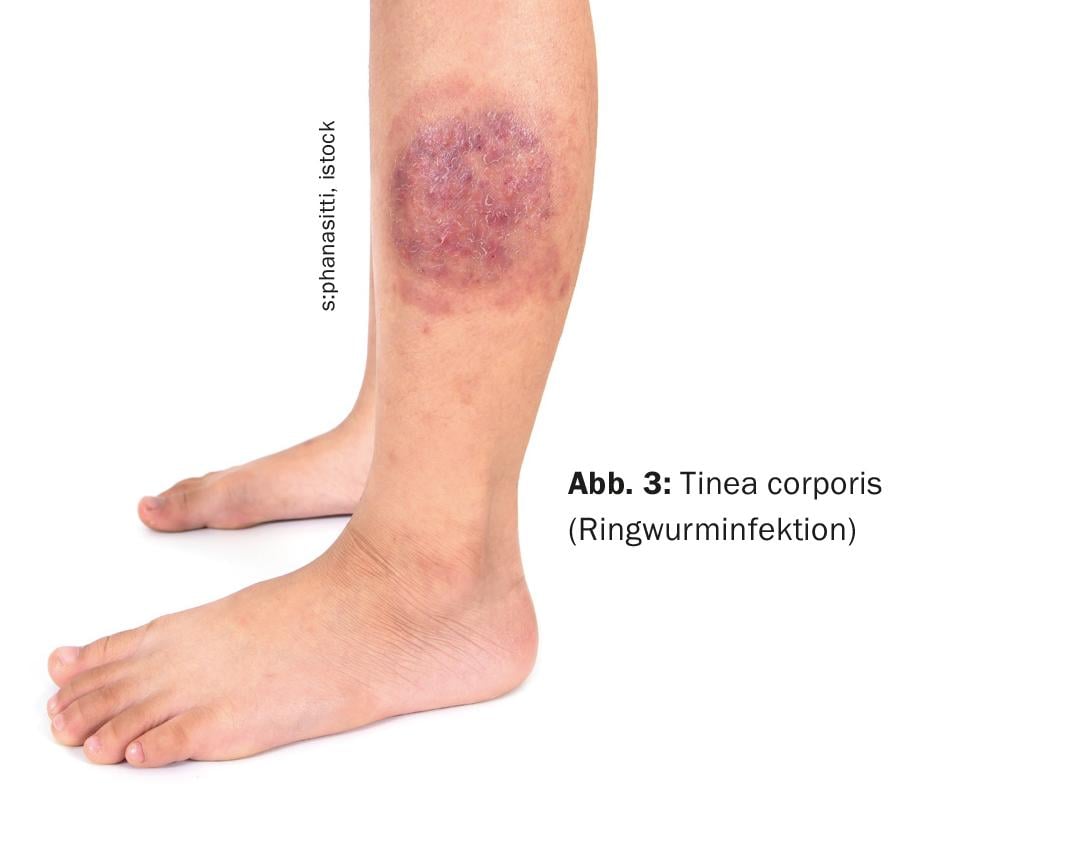
Tinea corporis (Fig. 3) appears on the exposed skin of the legs, arms, or upper body as centrifugally growing, erythrosquamous scaly plaques (“ringworm”). In adults, the causative agent is often T. rub-rum, besides T. interdigitale. In children, it is often zoophilic dermatophytes transmitted by small furry animals, e.g. M. canis from cats as the main host.
Tinea capitis: The symptoms of this fungal infection of the hairy scalp range from discrete scaling to circumscribed hair loss (hyperkeratotic form), to purulent abscesses (kerion celsi) due to deep infection of the hair roots [2]. Children are most often affected. Accumulations may occur in kindergartens and schools, with an increasing incidence observed in recent decades. In Austria, the predominant pathogen is M. canis (85%), with Trichophyton species also occurring. In the case of M. canis, infection is confined to the surface of the hair (Ectothrix); in contrast, the mycelium of most Trichophyton species invades the hair shaft (Endothrix). Under a Wood lamp, Ectothrix fluoresces but Endothrix infection does not.
Onychomycosis: A fungal disease of the nails affects large parts of the population; the prevalence in Western Europe is estimated at 10 to 20 percent. The incidence increases with age. Onychomycosis is particularly common in patients with diabetes mellitus; here the disease is not only a cosmetic problem, but can result in an infection that threatens the limb. Immunosuppression, tinea pedis and psoriasis are also risk factors. Childhood nail fungus used to be a rarity, but is now becoming more common. Dermatophytes are the most common pathogens of onychomycosis; it is then called “tinea unguium”. Most important here is T. rubrum, besides T. interdigitale and others. Other causative agents are shoot or mold fungi; multiple infestations with different fungi are also possible. Fungal infection leads to hyperkeratotic thickening and yellow-brown discoloration of the nail and finally to onycholysis; toenails are more frequently affected than fingernails.
Diagnosis of dermatomycoses
The basis for effective treatment is species-specific and timely identification of the pathogen. A visual diagnosis is not sufficient for this purpose. Rather, a mycological diagnosis is required. In addition, the monitoring of the pathogen spectrum is of great relevance for the assessment of the epidemiological situation and the prevailing infection routes. One example is the causative agents of tinea capitis in children, which differ according to geographic region and whose incidence in Europe is changing compared to earlier years, e.g., also due to migration from Africa. Another example is the more frequent occurrence in recent years of the zoophilic dermatophyte A. benhamiae, transmitted from guinea pigs and others, which causes inflammatory tinea in children and adolescents that requires timely systemic treatment [3].
Material collection: Skin scales or nail shavings must be collected for diagnosis. Any topical treatments should have been applied at least 14 days ago at the time of sample collection. After wiping the collection site with 70% alcohol, scales are scraped from the active border zone of the lesions, e.g., with a sterile scalpel; hairs are epilated. Nail material is obtained from the nail bed using a burr, from the discolored, thickened and crumbling portion of the nail from the underside of the nail. The material is transported in sterile, preferably light-protected containers without medium at room temperature. In tinea capitis, head scales are obtained by scraping with a scalpel, as well as hair roots with tweezers. For screening during an outbreak, it is also suitable to use a brush that is pressed directly onto the fungal culture medium after brushing through it [4].
Microscopic detection: This is done by microscopy of a native preparation with 20% potassium hydroxide solution. This makes skin, hair and nail particles transparent after one to two hours, and the enclosed fungal hyphae and possibly spores then become microscopically visible due to stronger light refraction. More sensitive and faster, however, is fluorescent staining, e.g. with Calcofluor White or Blankophor, which bind to chitin-containing structures that fluoresce bright white under UV light.
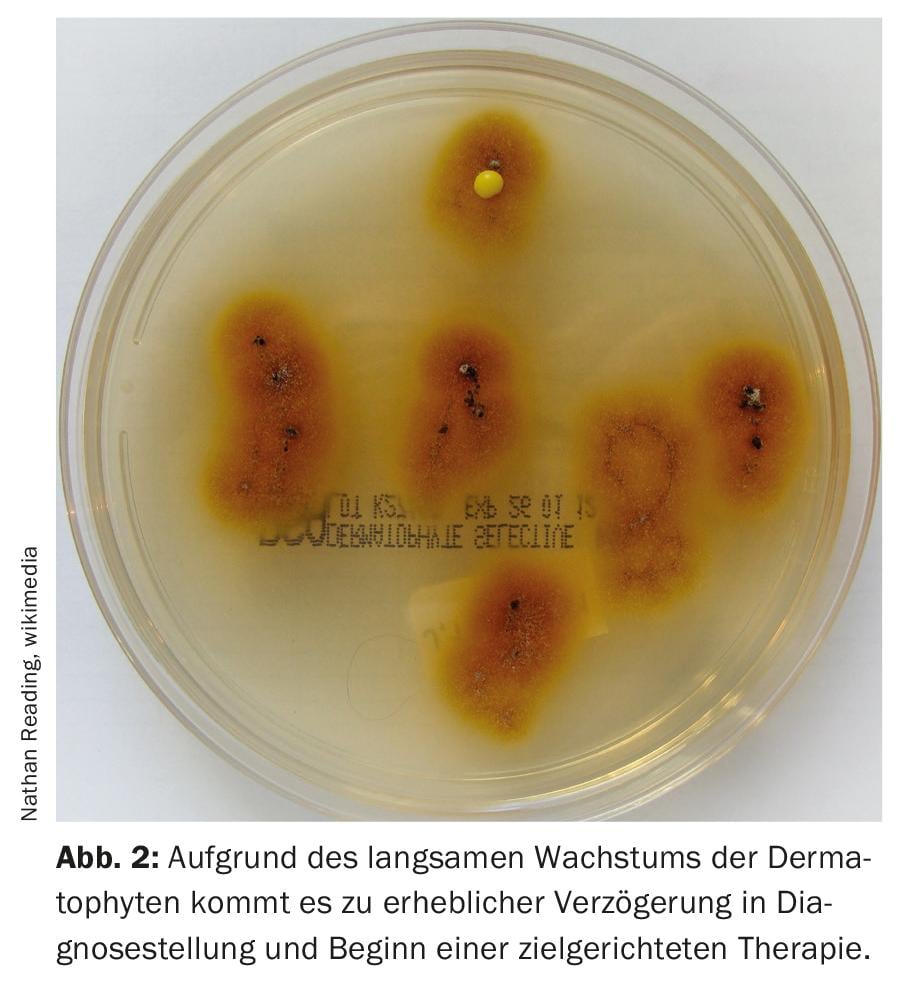
Culture-based detection: Culture-based pathogen identification is the current reference standard. Two plates of each sample are inoculated with nutrient agar (e.g., Sabouraud dextrose agar), one of which is inoculated with cycloheximide to suppress mold growth. The plates are incubated at 28°C for at least three weeks. For slow-growing dermatophytes, cultures require four to six weeks. Identification and differentiation are performed for dermatophytes and molds based on macro- and micromorphological characteristics. One challenge in culture diagnostics is that morphological features of fungi essential for species identification are not always formed, making differentiation difficult, especially in closely related species. A modern method that can be used for the analysis of dermatophytes like the shoot and mold fungi is the identification of typical metabolites or peptide patterns from the pure culture of the fungi by MALDI-TOF mass spectrometry.
Due to the slow growth of dermatophytes, there is a considerable delay in diagnosis and initiation of targeted therapy. In addition, cultural detection often yields a false negative result, especially for onychomycoses; up to 50 percent of the pathogens do not grow in culture, probably mainly due to the use of over-the-counter antifungal agents even before the first visit to the doctor. In acute infections such as tinea capitis, delayed treatment means increased potential for spread in addition to patient distress. Due to the drawbacks of conventional techniques, molecular methods have gained importance in recent years.
Molecular biological dermatophyte detection [5,6]: By means of PCR (Polymerase Chain Reaction) sequences from the genome of the fungi are amplified exponentially. This allows more specific and sensitive detection than conventional methods; even pathogens that are growth-inhibited by antifungal treatment are detected. PCR methods for the detection of dermatophytes, and likewise for some shoot and mold fungi from nail and skin material, are available in the form of commercial kits; some of these use conventional PCR, but some use the more modern real-time PCR. PCR analysis increases the proportion of positive results and drastically reduces the time to diagnosis, as results are available within 24 to 48 hours. The first PCR kits available only allowed universal detection or detection of a few genera; more recent multiplex kits allow more extensive species-specific diagnostics, at least for dermatophytes encountered in routine diagnostics. However, due to the often close phylogenetic relationship, the reliable differentiation of species remains a challenge in some cases. By its nature, a PCR kit can only detect the pathogens for which it is designed; thus, new or rare pathogens may be missed. On the other hand, PCR methods are so sensitive that false-positive, possibly implausible results can also occur; PCR results must therefore be assessed with clinical expertise. Even more specific and comprehensive than previous multiplex PCR assays is a microarray assay that has been on the market since 2018 [7]. Twenty-three common and rare pathogenic dermatophytes and a selection of six non-dermatophyte species are detected. In practice, the selection of the appropriate PCR kit will depend on the research question. In the case of cutaneous mycoses, the pathogen must be identified from among about a dozen common dermatophytes; in the case of onychomycosis, only T. rubrum and T. interdigitale are generally considered as pathogens among the dermatophytes, in addition to yeast or molds. In addition, the advantages of PCR diagnostics will have to be weighed against the importance of rapid diagnosis and the cost of reagents, equipment, and labor [8]. Due to the not inconsiderable costs of analysis, this diagnostic method is often reserved for selected questions; it is currently not practicable for routine use.
Therapy of dermatophytoses
Today, drug treatment of dermatophyte skin infections shows excellent results, with cure rates of 80 to 90 percent [9]. In recent clinical trials, complete cure is the clinical endpoint. The topical and oral formulations of antifungal agents available today are effective in the majority of patients when used regularly and for the prescribed duration. Generally, topical treatment is used for localized infections and oral therapy is used for more extensive infestations.
Topical antifungals: After microscopic detection of a fungal infection, topical treatment can be started directly. To prevent recurrences, topical therapy should be continued for three to four weeks beyond clinical healing to ensure that dormant fungal spores are eliminated with the upper layers of the stratum corneum [10]. Lack of treatment adherence is often observed with topical therapies and may jeopardize treatment success. A selection of different antifungal agents is shown in Table 1 . Azoles are well established in the treatment of dermatophytoses. They are usually offered as a 1% formulation (cream, solution or spray). It should be noted that the spectrum of activity of different azoles differs in detail: for example, clotrimazole is effective against T. rubrum but not against other common Trichophyton species (T. mentagrophytes, verrucosum, interdigitale) [17]. An effective alternative is the topical cream formulation with 1% terbinafine; with this, remissions can be achieved after a short time in some cases (e.g., interdigital tinea pedis) . In addition, there is a 1% film-forming terbinafine solution for single application that showed high efficacy in T. pedis in a controlled study [11]. Most antifungal agents exhibit predominantly fungistatic effects against dermatophytes, whereas terbinafine has fungicidal effects, including against dormant forms of dermatophytes. Azoles and cyclopirox are effective as broad-spectrum antifungals against dermatophytes, yeasts and molds. Terbinafine has a weaker effect on infections with yeasts (e.g., Candida).
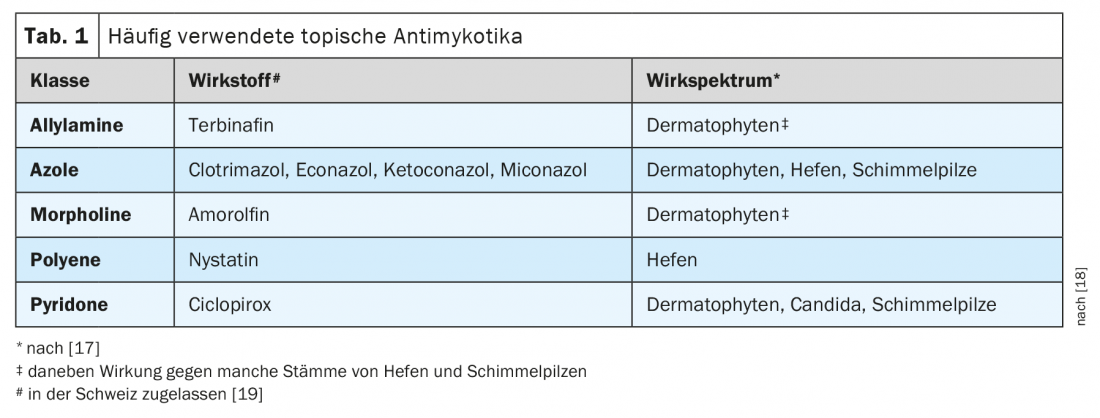
Local combination therapy for inflammatory dermatophytoses: Dermatophytosis is often accompanied by significant inflammatory changes. Therefore, both effective control of the pathogen and suppression of inflammation appear to be reasonable, and the use of topical combination preparations containing corticosteroids is recommended [12].
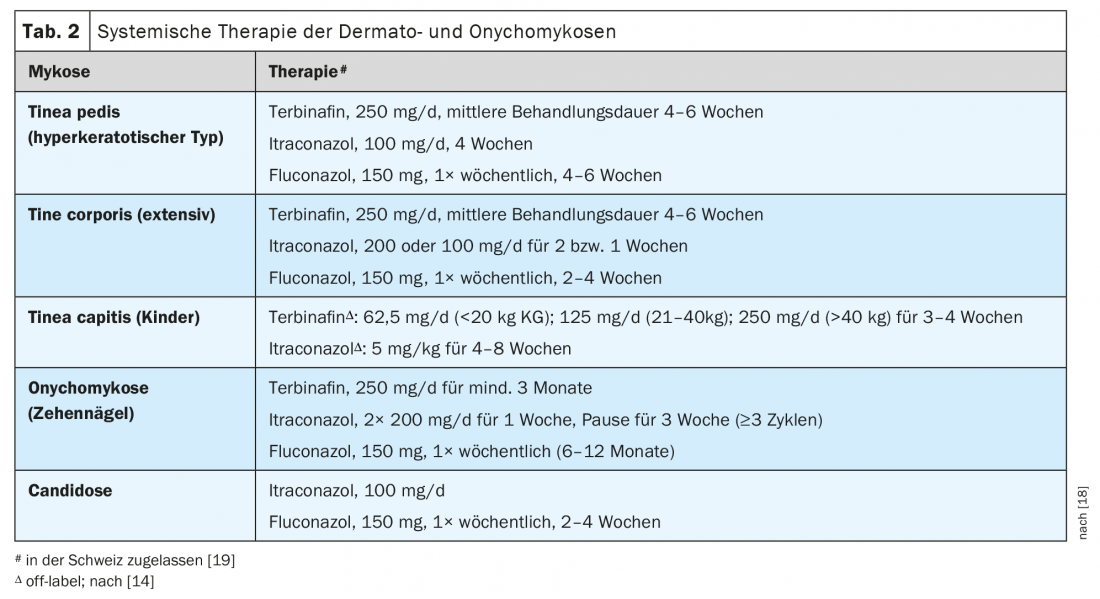
Systemic therapy: If topical therapy is ineffective, systemic treatment is required. It is also indicated, for example, in cases of tinea corporis with large extension or multiple foci, hyperkeratotic tinea pedis, and tinea capitis. Only when a positive culture or molecular biological result is available should systemic therapy be started if indicated. For the oral therapy of dermatophytoses such as tinea pedis or tinea corporis, terbinafine and itraconazole are available in the first line (Tab. 2); with them, rapid and long-lasting remissions are achieved. Terbinafine is preferred as the first-line agent for Trichophyton infections, and itraconazole for microsporiasis. Fluconazole is a second-line alternative. In general, local or systemic side effects of antifungals are not a significant problem. Commonly used terbinafine is a safe drug, but potential interactions with certain drugs should be considered; rarely, induction of psoriasis may occur [13]. Itraconazole, as an inhibitor of cytochrome P450 enzymes, can lead to numerous interactions with other drugs. Especially in the presence of pre-existing liver disease, the use of systemic antifungals must be carefully considered. In the case of ketoconazole, an increased incidence of hepatitis was observed, which is why the drug is no longer used for oral therapy of superficial mycoses.
Therapy of tinea capitis
The therapy of tinea capitis in childhood is still a challenge. Treatment must always be systemic and adjuvant topical [14]. Terbinafine and the azoles itraconazole and fluconazole can be used as oral antifungal agents (Table 2). Terbinafine is effective in the case of infection with Trichophyton species, but less so in Microsporum infection, where the treatment duration or dosage may need to be increased. Secondarily, itraconazole and also fluconazole can be used, also in “off-label use”. Dosage suggestions can be found in Table 2. Therapy should only be terminated after negative culture success. To prevent spread of infection, a ketoconazole or selenium sulfide shampoo should be used in addition to oral therapy; asymptomatic carriers of infection (proven or suspected) should also be treated, at least topically [2]. Adjuvant measures such as shortening of hair and disinfection of inanimate materials are also indicated. By means of culture or PCR diagnostics, it must also be clarified whether a zoophilic infection is present in order to initiate antifungal therapy of domestic animals if necessary. Infected animals should definitely be treated as well, even if they are asymptomatic. Children who receive appropriate systemic and adjuvant topical therapy can return to school or kindergarten immediately. However, a one-week grace period should be observed for infections caused by anthropophilic pathogens [14].
Therapy of onychomycosis
A nail fungus infection has no self-healing tendency, but must always be treated. Cultural diagnosis is essential for successful therapy. An important prerequisite for successful therapy is also the patient’s willingness to undergo consistent treatment over a long period of time.
Topical treatment is indicated for white superficial nail infection (Leukonychia trichophytica) and distolateral subungual onychomycosis, provided that co-infestation of the nail matrix is excluded and there is no significant thickening of the nail plate [15]. Topical monotherapy is generally effective only for low nail fungal infestation (<50% of a nail plate). In addition, adherence to prolonged topical therapy regimens is often poor.
Ciclopirox and amorolfine are available as nail polishes; they must be applied daily and weekly, respectively, for nine to twelve months in the case of toenails. However, the rates of complete cure with monotherapies are considered low compared to systemic therapies or combination treatments.
Systemic treatment is indicated for all other forms and severities of onychomycosis. Especially in advanced onychomycosis, sustained success is most reliably achieved with oral therapy. Unambiguous fungal detection (cultural or by PCR) is required before systemic treatment [3]. In the case of nail mycoses caused by dermatophytes (approx. 80 percent of cases), a number of therapeutic options are then available (Tab. 2):
- Terbinafine: Treatment lasts at least three to six months. In mixed infections with yeasts, therapy is indicated only if there is a response in the first two to three weeks. Many studies show the highest cure rates for terbinafine. The rates of complete and mycological cures are 38 and 70 percent, respectively [9]. Improved cure rates also result when oral terbinafine is combined with amorolfine nail polish or ciclopirox nail polish.
- For pulse therapy with itraconazole , daily for one week. 2× 200 mg given, followed by three weeks off therapy; this is repeated in at least three cycles. This regimen prevailed over the continuous administration of itraconazole (200 mg/d for three months).
- Fluconazole as second-line therapy is dosed at 150 mg once a week. Treatment must be continued until healing (in studies, a mean of 9.3 months in the case of toenails).
Candida onychomycosis must be treated with yeast-active substances, e.g. fluconazole or itraconazole. For therapy of onychomycosis caused by the mold Scopulariopsi brevicaulis, itraconazole and terbinafine have been shown to be well effective. However, other onychomycoses caused by molds such as Aspergillus species often do not respond to conventional therapeutic regimens. Atraumatic nail removal has proven to be an adjuvant measure, e.g. by milling out the nails, by keratolysis with urea preparations under occlusion or – as the latest procedure – also by laser ablation. Surgical nail extraction is obsolete. To avoid reinfection, any tinea pedis that may be present should also be treated. To prevent recurrences, permanent topical follow-up treatment (preferably with a nail polish) may also be given. Patients can be advised to wear bathing shoes also at home; to disinfect shoes regularly (e.g. clotrimazole spray); to wash socks, underwear and towels at 60°C possibly with hygiene rinse [16]. Various non-drug treatments for onychomycosis (e.g., laser therapy and photodynamic therapy) have been described; however, there is still no conclusive evidence of long-term efficacy for these from stringently controlled studies.
Therapy of Candida infections of the skin
Opportunistic candidiasis of the skin occurs primarily in intertriginous areas or in warm, moist environments (as in diaper dermatitis and in incontinent bedridden patients). They respond well to topical antifungal agents. The azoles (econazole, clotrimazole, ketoconazole, miconazole), as well as the polyenes, have proven effective ( Tab. 1) . For oral treatment, fluconazole or itraconazole is usually used (Table 2). Pityriasis versicolor caused by Malassezia infectionis usually treated topically with azoles, but the allylamines are also effective here. For extensive or persistent infections, and especially for Malassezia folliculitis, oral treatment with itraconazole or fluconazole may also be used.
Take-Home Messages
- Fungal infections are the most common diseases of the skin and its appendages. They are mostly caused by dermatophytes, but also by fungi and, less frequently, by molds. Improved diagnostics, adapted therapy and prevention can increase the rates of permanent cures.
- The basis for effective treatment is species-specific and timely identification of the pathogen. A visual diagnosis is not sufficient.
- In addition to the classical microscopic and cultural detection, molecular biological dermatophyte detection has gained importance. Even more specific and comprehensive than previous multiplex PCR tests is a microarray assay that has been on the market for a few years.
- The topical and oral antifungal therapies available today are effective in most patients when used regularly and as prescribed. While topical treatment is usually sufficient for localized infections, oral therapy is indicated for more extensive infestations.
Literature:
- Wiegand C, et al: Dermatologist 2019(70): 561-574.
- Hay RJ: Mycopathologia 2017(182): 87-93.
- Nenoff P, et al: Dermatologist 2016(67): 676-679.
- Mayser P: Dermatologist 2019(70): 594-600.
- Kupsch C, et al: Dermatologist 2019(70): 627-637.
- Begum J, et al: J Basic Microbiol 2020(60): 293-303.
- Uhrlass S, et al: Dermatologist 2019(70): 618-626.
- Verrier J, et al: Mycopathologia 2017(182): 193-202.
- Hay R: J Fungi 2018; 4. pii: E99.
- Guideline Tinea of the Free Skin, No. 013/002, 2008,
- Ortonne JP, et al: J Eur Acad Dermatol Venereol 2006(20): 1307-1313.
- Czaika VA, et al: Dermatologist 2015(66): 360-369.
- Dürrbeck A, et al: Dermatologist 2016(67): 718-723.
- AWMF: Guideline Tinea capitis, No. 013/033, 2019
- AWMF: Guideline Onychomycosis, No. 013/003, 2010
- Hasche EG, et al: Dermatologist 2018(69): 718-725.
- Tietz HJ: Deutsche Apothekerzeitung 2011(20): 70.
- Austria-Codex, https://austria-codex.at (last accessed 15.11.2021)
- Swissmedic, www.swissmedicinfo.ch (last accessed Nov. 15, 2021).
DERMATOLOGY PRACTICE 2021; 31(6): 4-9