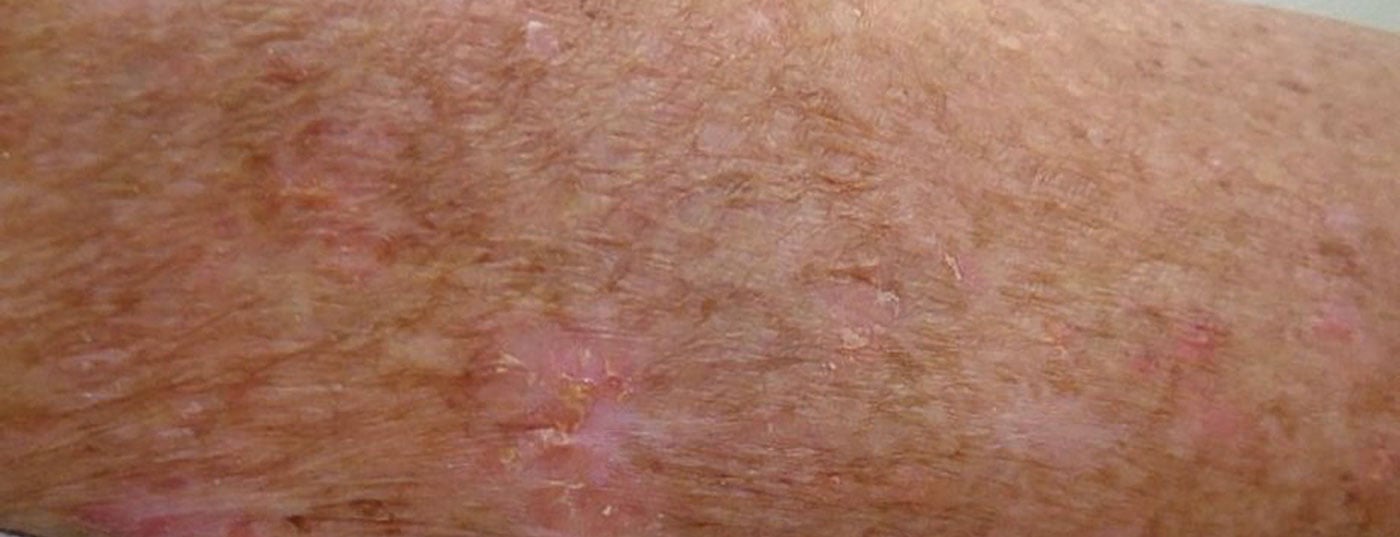
Actinic keratoses are the most common precancerous lesions on the skin. Although only five to ten percent of actinic keratoses develop into skin cancer, it is not possible today to predict with certainty which lesions will progress to invasive squamous cell carcinoma and which will not. Therefore, experts recommend treating actinic keratoses in general. The broad spectrum of treatment options has recently expanded to include an innovative drug therapy option.
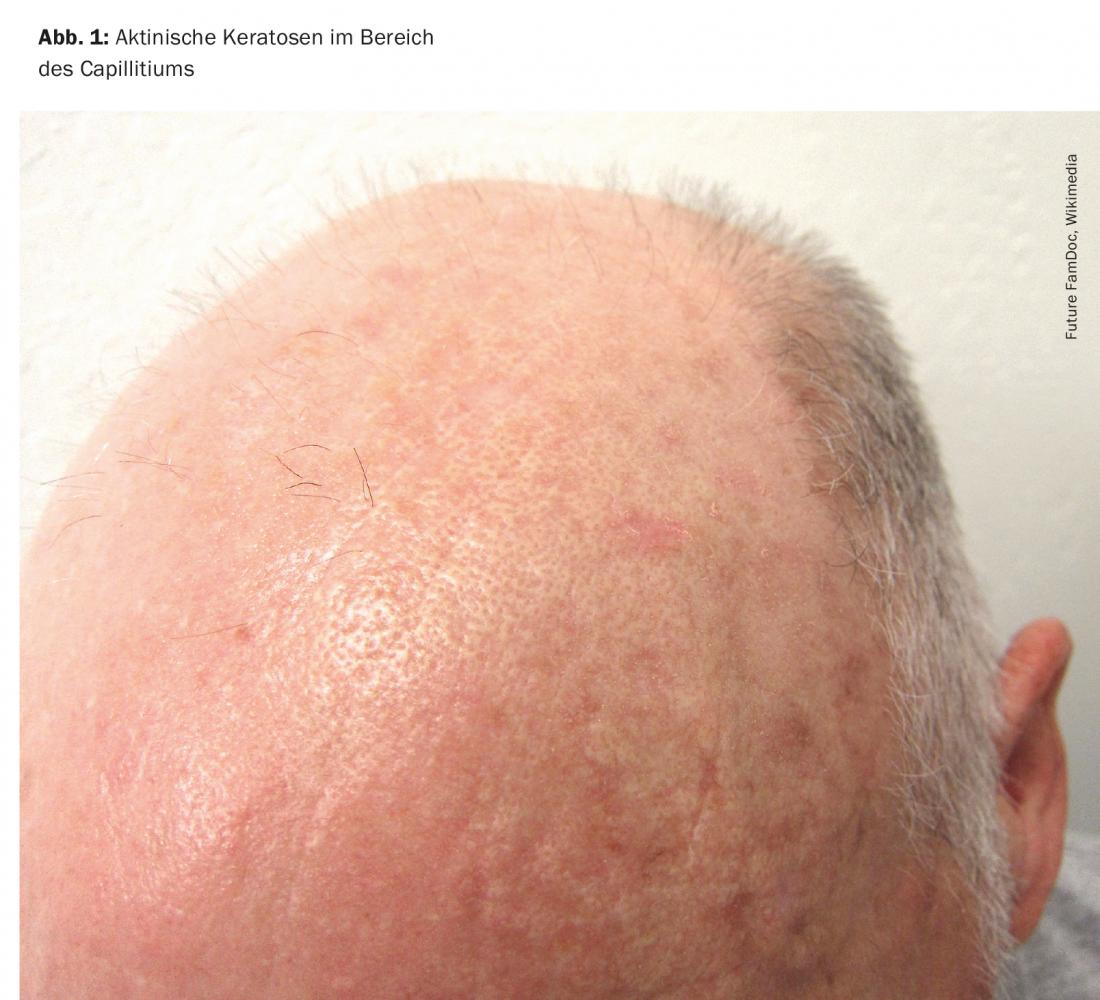
Actinic keratoses are primarily caused by cumulative damage to the skin from ultraviolet radiation. UV radiation induces loss-of-function mutations of the tumor suppressor gene p53. Consequently, uncontrolled proliferation of the degenerated keratinocytes occurs. The risk groups for the development of actinic keratoses include people with light skin types who spend a lot of time outdoors in their free time or for occupational reasons. Predilection sites are all light-exposed areas of the skin, especially the hairless scalp (Fig. 1), the ear helices, as well as the forehead, bridge of the nose, cheeks, lower lip (cheilitis actinica), but also forearms and backs of the hands.
Prevalence increase predicted
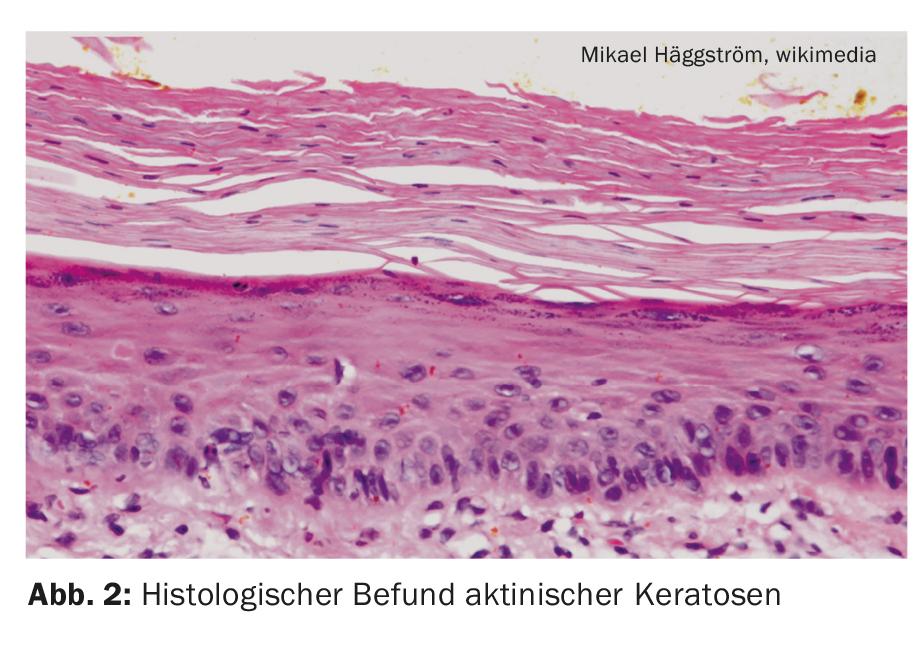
According to epidemiological data, actinic keratoses affect 40-60% of Australian and 38% of European adults, with an increasing prevalence [1,2]. The incidence increases in an age-correlated manner, the peak of the disease is beyond the age of 50, although younger patients may also be affected depending on the duration and intensity of light exposure. Histologically, actinic keratoses are confined to the epidermis, but morphologically and genetically they resemble invasive squamous cell carcinoma. In the absence of treatment, actinic keratoses may progress through the basement membrane into the dermis and mutate into an invasive growing squamous cell carcinoma. Approximately ten percent of all actinic keratoses exhibit such a transition [3].
Large therapy arsenal available
Diagnostically, dermoscopy, confocal laser microscopy, and optical coherence tomography can be used, especially when clinical examination and inspection reveal unclear findings [4]. Various lesion- and field-directed therapeutic options are available for the treatment of actinic keratoses. An advantage of field-directed procedures is that multiple contiguous and also subclinical, i.e., not yet visible or palpable, lesions are also treated [5]. Topically applied active ingredients in solution, gel or cream form are used. The primary field-targeted topical medications approved in Switzerland include diclofenac, 5-fluorouracil, imiquimod, aminolevulinic acid, methylaminolevulinate and, more recently, tirbanibulin – a novel agent from the tubulin inhibitor group for the external treatment of actinic keratosis. For example, lesion-directed therapy procedures may include shave excision, cryosurgery, or laser ablation.
Novel topical “first-in-class” microtubule inhibitor.
The approval of Klisyri® (tirbanibulin) [12] represents a significant advance in the treatment of actinic keratoses due to its short treatment regimen – once-daily application for 5 days – and proven efficacy and safety profile [6]. Topical microtubule inhibitor is indicated for the treatment of Olsen I lesions on the face or scalp in adults. The selective antiproliferative mechanism of action of tirbanibulin is based on blockade of the intracellular protein tyrosine kinase Src, which is increasingly expressed in actinic keratoses and plays a role in progression to PEK [7]. The limitation to a maximum of 25 cm2 skin surface has nothing to do with toxicity, but can be explained by empirical-methodical reasons, according to Prof. Dr. med. Thomas Dirschka, CentroDerm, Wuppertal (D) [8]. Since complete healing (“complete clerance”) was almost impossible without delineation of the skin area, the healing rate was defined in relation to the skin area for the clinical studies. The approval of tirbanibulin is based on the two multicenter double-blind phase III studies, KX01-AK-003 and KX01-AK-004 [9]. A total of 702 patients with actinic keratoses on the face or scalp were randomized into each of two groups: tirbanibulin 1% vs. vehicle were randomized. Patients applied the substance once daily for five consecutive days to a 25 cm2 skin area with four to eight lesions. The primary endpoint was complete disappearance of all lesions by day 57. Both phase III studies met their primary endpoint with statistical significance versus vehicle (p<0.0001). In the KX01-AK-003 study, complete clearance was observed in 44% of tirbanibulin-treated patients compared to 5% in the placebo group. In the KX01-AK-004 trial, the corresponding figures were 54% with tirbanibulin versus 13% with vehicle. Local reactions were mostly mild to moderate erythema, scaling, itching, and pain at the application site that resolved spontaneously [10].
Congress: European Association of Dermato-Oncology (EADO)
Literature:
- Green AC: Epidemiology of actinic keratoses. Curr Probl Dermatol 2015; 46: 1-7.
- Schaefer I, et al: Prevalence and risk factors of actinic keratosis in Germany. JEADV 2014; 28(3): 309-313.
- European Skin Cancer Foundation: Actinic keratosis, www.escf-network.eu/de/patienten/hautkrebs/aktinische-keratose.html (last accessed May 24, 2022).
- S3 guideline Actinic keratosis and squamous cell carcinoma of the skin, long version 1.1.-March 2020, AWMF register number: 032/022OL
- Gutzmer Ralf, et al: Actinic keratosis and cutaneous squamous cell carcinoma. Possibilities of therapy, Dtsch Arztebl Int 2019; 116: 616-626.
- “Almirall receives European Commission approval of Klisyri® (tirbanibulin), an innovative topical treatment for actinic keratosis,” Almirall, July 19, 2021.
- Borik-Heil L, Geusau A: Actinic keratoses. close up 2021; 20: 45-55.
- Dirschka T: Management of Actinic keratosis and field cancerication, Prof. Dr. med. Thomas Dirschka, EADO Annual Meeting, 21-23.04.2022.
- Blauvelt A, et al: Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N Engl J Med 2021; 11; 384(6): 512-520.
- Worldometer. Population of Europe. 2020, www.worldometers.info/world-population/europe-population (last accessed 05/24/2022)
- S3 Guideline Prevention of Skin Cancer, Version 2.1 – September 2021, AWMF Register Number: 032/052OL
- Drug Information, www.swissmedicinfo.ch (last accessed May 24, 2022).
DERMATOLOGIE PRAXIS 2022; 32(3): 30-32 (published 6/15/22, ahead of print).